FibroScan®, the non-invasive gold standard solution for liver health management
4.200+ peer-reviewed publications & 180+ international guidelines
- FibroScan® enables non-invasive diagnosis and monitoring at the point-of-care.
- FibroScan® is powered by three unique, patented and validated biomarkers to quickly and easily assess liver health.
Interested in discovering more about FibroScan®? Please, fill out the form. We will contact you shortly to discuss your needs and advise you on the most suitable device.
-
Contact us to discover more
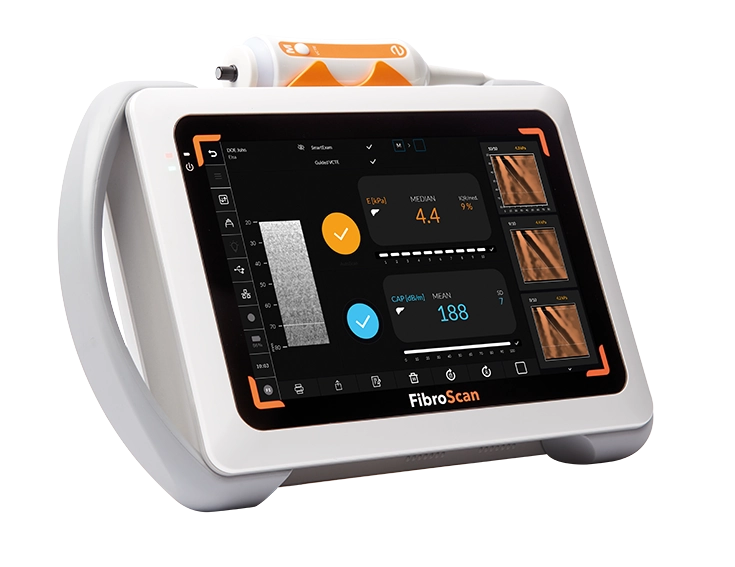
FibroScan® Mini+ 430 is light and easy to handle, designed for multi-center sharing.
Powered by LSM by VCTE™ and CAP™ for liver fibrosis and liver steatosis assessment. -
Contact us to discover more
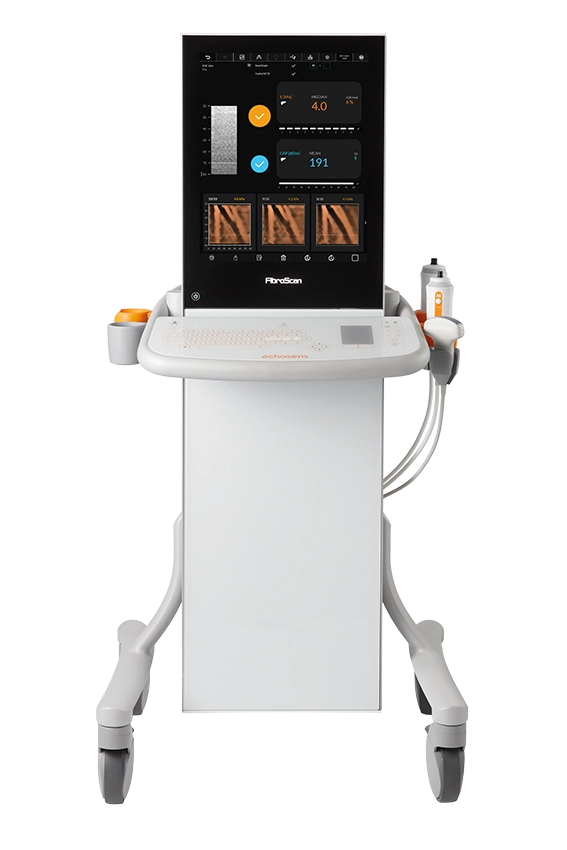
From cart-based to transportable, FibroScan® Compact 530 is the optimal liver assessment solution for any point-of-care setting.
Powered by LSM by VCTE™ and CAP™ for liver fibrosis and liver steatosis assessment. -
Contact us to discover more
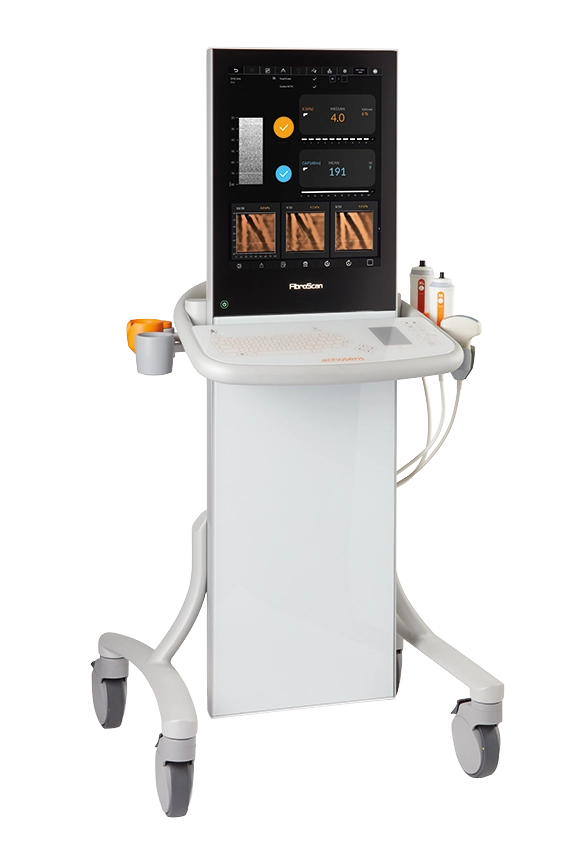
FibroScan® Expert 630 is the complete solution for advanced liver disease management with its capability to measure the spleen stiffness. It embeds an ultrasound localization probe for both liver and spleen targeting.
Powered by LSM by VCTE™, CAP™ and SSM by VCTE™ as part of an overall assessment of the liver. -
Contact us to discover more
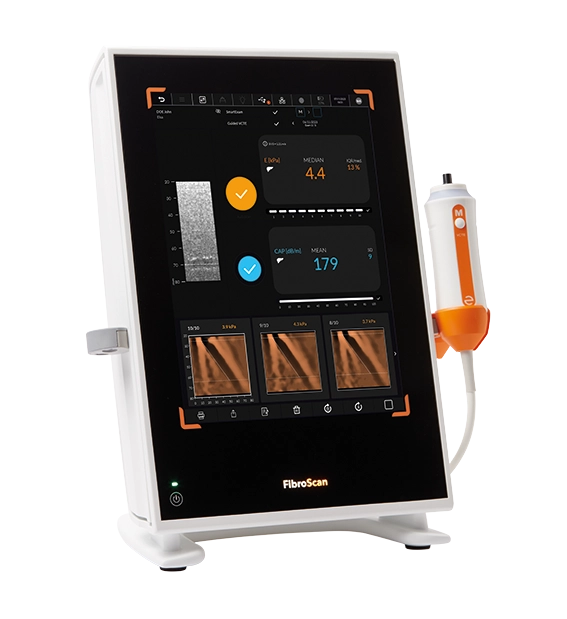
FibroScan® GO is an affordable and cost-effective pay-per-exam solution with all services included to improve liver health management.
Powered by LSM by VCTE™ and CAP™ for liver fibrosis and liver steatosis assessment.

LSM: Liver Stiffness Measurement / SSM: Spleen Stiffness Measurement / CAP™: Controlled Attenuation Parameter / VCTE™: Vibration-Controlled Transient Elastography
Products in the FibroScan® range are class IIa medical devices according to Rule 10 of ANNEX VIII of Regulation EU 2017/745 (CE 0459) and are manufactured by Echosens™. FibroScan® devices are intended to provide: Liver stiffness measurements at a shear wave frequency of 50 Hz, liver ultrasound attenuation measurements (CAP: Controlled Attenuation Parameter) at 3.5 MHz and spleen stiffness measurements at a shear wave frequency of 100 Hz (FibroScan® 630 Expert only). These non-invasive devices are intended to aid clinical management, diagnosis, and monitoring of patients with confirmed or suspected chronic liver disease, as part of an overall assessment of the liver. FibroScan® devices may aid the healthcare professionals in the assessment of liver fibrosis, steatosis, and in determining the likelihood of cirrhosis, and its complications (FibroScan® 630 Expert only). They are used, in conjunction with other clinical and laboratory data, during liver assessment in patients with confirmed or suspected chronic liver disease. Examinations with FibroScan® devices shall be performed by an operator who has been certified by the manufacturer or its approved local representative. Operators are expressly recommended to carefully read the instructions given in the user manual and on the labelling of these products. Check cost defrayal conditions with paying bodies. This marketing material is not intended for US audience. © 2024 Echosens - Echosens™, the Echosens logo, FibroScan® and the FibroScan logo are trademarks owned by Echosens SA. All rights reserved.