FibroScan® Solutions in MASH* Clinical Trials
FibroScan® and its associated solutions can be leveraged to significantly reduce screen failure rate, accelerate patient enrollment, and monitor therapeutic response.
Download the White Paper and enjoy the reading!
*Formerly known as NASH

Our solutions can support the development of MASH therapies
with the reference liver non-invasive point-of-care testing solutions
- Accelerating drug development,
- De-risking trial execution
- Preparing for commercialization
Overview of the white paper
Schematic of a typical Phase 3/4 clinical trial for accelerated drug approval
to treat patients with non-cirrhotic MASH.
(EOT: End of Treatment, M: Month)
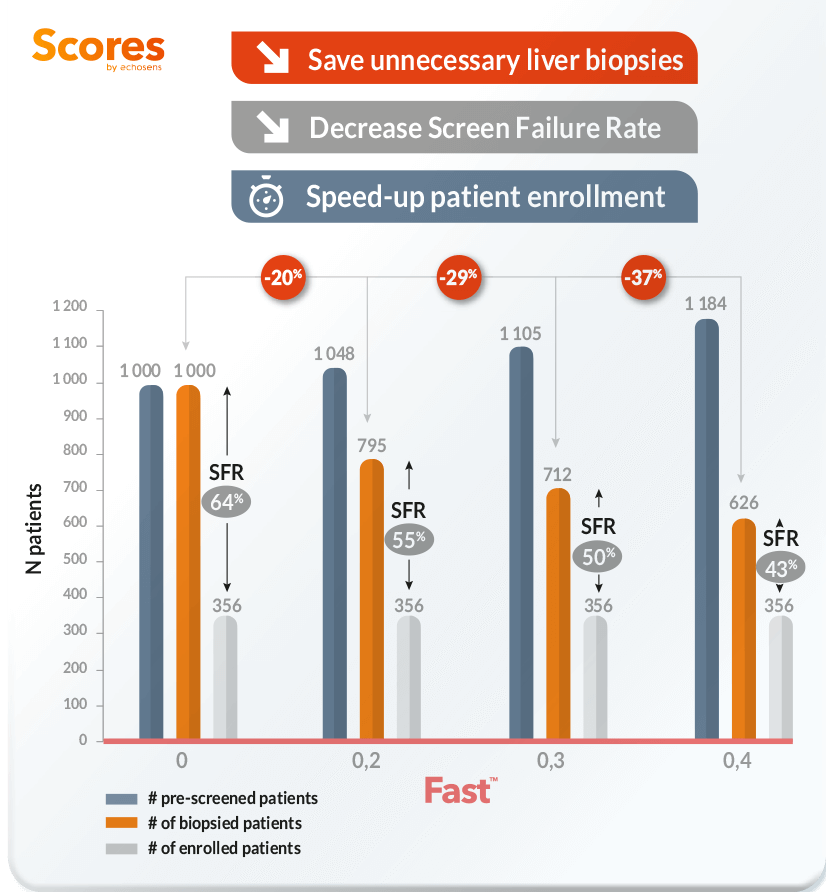
Impact of the use of Fast on patient enrollement
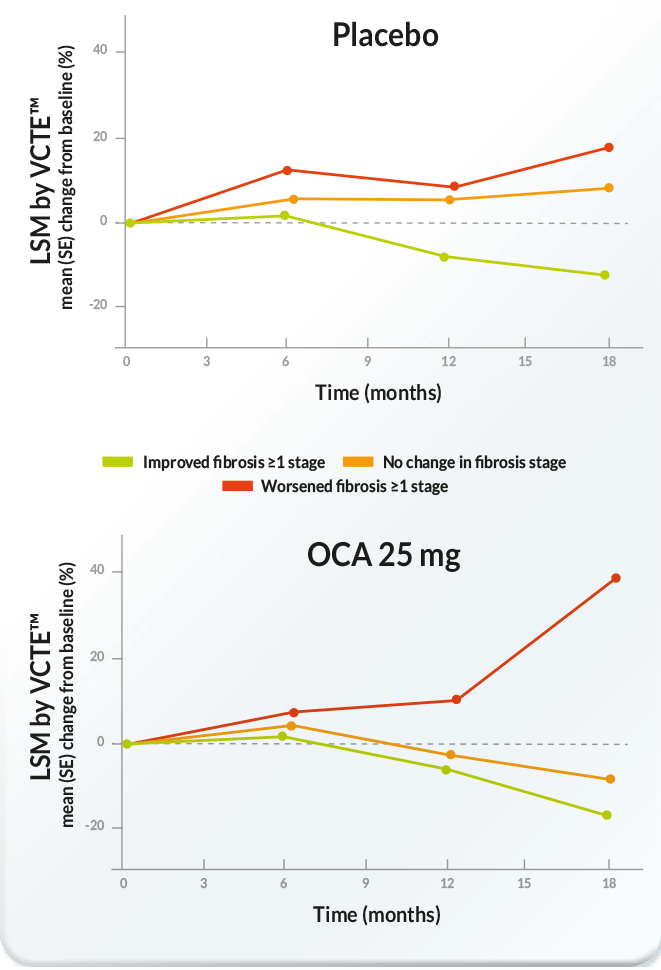
Mean percentage change from baseline in LSM by VCTE™ over time
by treatment group and histological fibrosis improvement status
(adapted from [1])
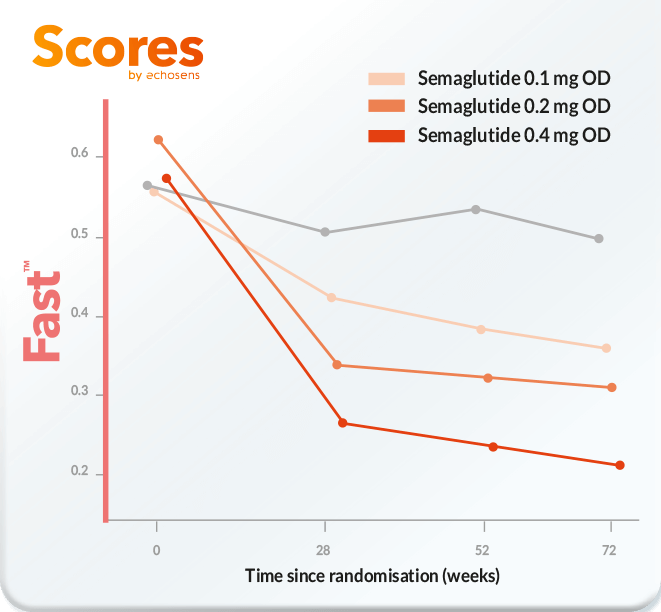
Change from baseline to week 72 of Fast™ score
Subcutaneous Semaglutide Phase 2b trial
(adapted from [2])
[1] – Rinella M, Dufour J-F, Anstee QM, Goodman Z, Younossi Z, Harrison SA et al. Non-invasive evaluation of response to obeticholic acid in patients with NASH: Results from the REGENERATE study. Journal of Hepatology 2021.
[2] – Wong VW-S, Quentin A, Geerts A, Mette K, Ladelund S, Ratziu V et al. Change in FibroScan-aspartate aminotransferase (FAST) score is associated with histological improvement in non-alcoholic steatohepatitis activity: The Journal of Hepatology; 2021.
The FibroScan® Family of Products (Models: 502 Touch, 630 Expert 530 Compact and 430 Mini+) is intended to provide shear wave speed measurements and estimates of tissue stiffness as well as ultrasound coefficient of attenuation (CAP : Controlled Attenuation Parameter) in internal structures of the body. The Shear wave speed and stiffness measurements may be used as an aid to clinical management of adult patients with liver disease. The FibroScan® Family of Products (Models: 502 Touch, 630 Expert 530 Compact and 430 Mini+) is indicated for non-invasive measurement in the liver of 50 Hz shear wave speed and estimates of stiffness as well as determining a 3.5 MHz ultrasound coefficient of attenuation (CAP: Controlled Attenuation Parameter). The shear wave speed and stiffness, and CAP may be used as an aid to diagnosis and monitoring of adult patients with liver disease, as part of an overall assessment of the liver. Shear wave speed and stiffness, and CAP* may be used as an aid in the clinical management of pediatric patients with liver disease. FibroScan® 630 (Expert) is also indicated for noninvasive measurement in the spleen of 100 Hz shear wave speed and estimates of stiffness that may be used as an aid to diagnosis, monitoring and clinical management of adult patients with liver disease, as part of an overall assessment of the liver.
*CAP for pediatric patients with liver disease is only available with SmartExam capability.