Our solutions for clinical trials
Our Expertise and Turn-Key solutions supporting each step of your trial and driving success

Accelerate clinical trial & optimize trial cost-effectiveness
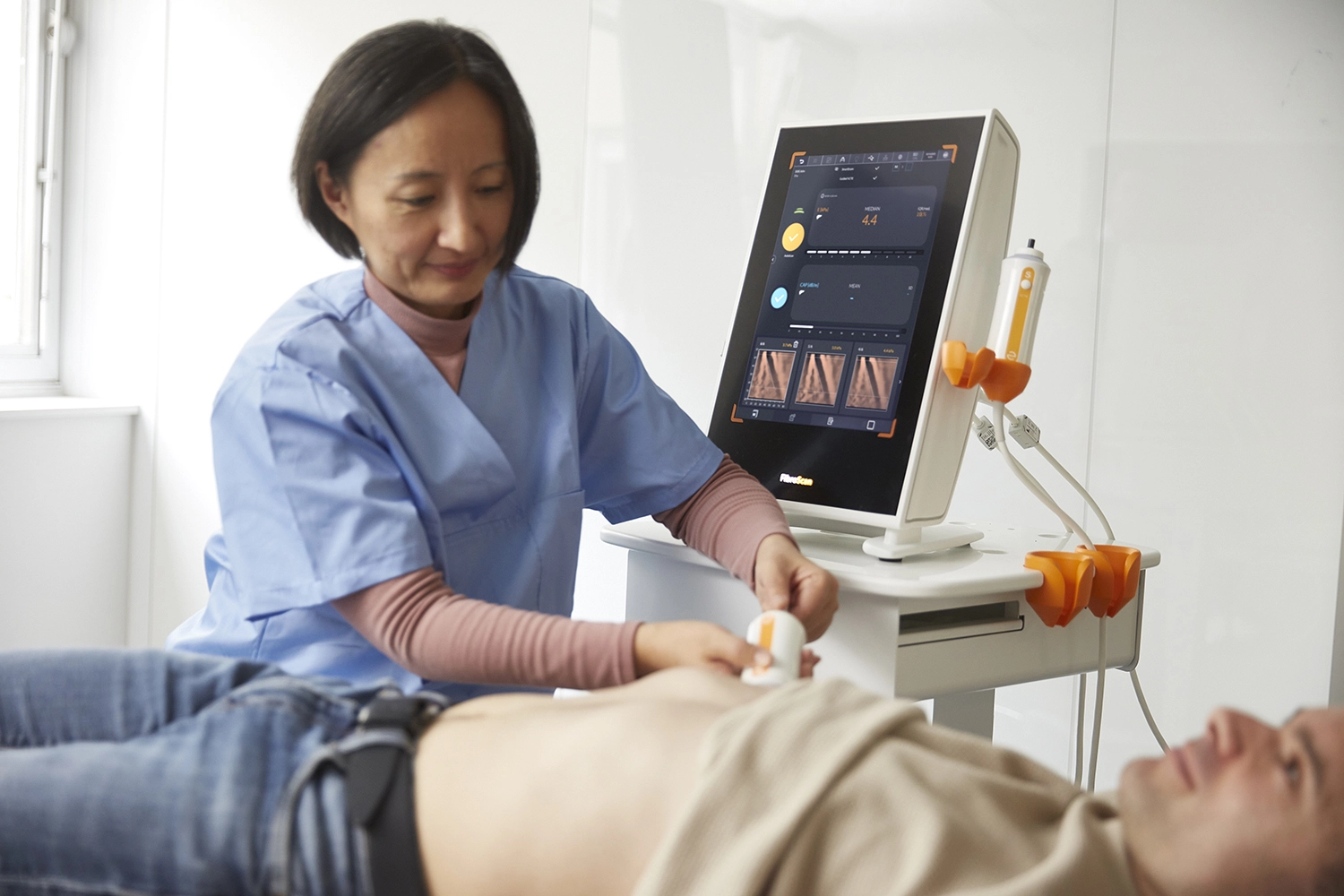
Echosens provides best-in-class, non-invasive tests for the liver and spleen, to help reduce screen failure rate and monitor treatment response.
FibroScan® accurately assesses presence of steatosis, fibrosis, cirrhosis & active MASH.
*Formerly known as NASH
De-risk trial execution
Following Echosens review, one-third of selected sites needed action(s) to be fully qualified.
We support you in standardizing the capabilities of FibroScan® and maximizing operator proficiency before the start of the trial.
During the trial, we use real-time analytics to closely monitor the FibroScan® fleet, ensuring data consistency.
Ensure data quality across all sites and operators
FibroScan® Exam Quality Control ensures data quality and consistency for all exams.
Data Management streamlines FibroScan® data collection and transmission.
Prepare for drug approval, market access and launch
The same solutions are used in clinical trials and in routine practice, which allows you to accelerate your market development.

Echosens your end-to-end partner for research
70+
clinical trials as NIT provider
up to 7
millions of exams per year
100+
countries equipped
23
years of clinical experience in liver
A set of solutions to support each step of your trial
Preparing for commercialization
Enlarge diagnostic capacity when your drugs hit the market with
a cost-effective test, associated workflow and established recognition.
Echosens is engaged with many sponsors in screening
and monitoring patients for clinical trials in MASLD/MASH.
More than 1,000 clinical institutions chose FibroScan® for their trials.
“The use of FibroScan® has tremendous value:
Once we have a patient on an FDA-approved treatment, we can potentially use FibroScan® to monitor response to therapy.
For those people that aren’t responding, we may stop the ineffective drug.
For those that are responding, we can provide positive reinforcement to continue to take their medication, remain compliant with their medication and effect a positive outcome that is potentially longstanding and will have a positive impact down the road on their long-term outcomes.
Finally, where we are able to utilize VCTE™ down the road, in the sense of predicting long-term outcome, that would be the Holy Grail and the hope for tomorrow.”
Dr. Stephen Harrison
|M.D., gastroenterologist and hepatologist, medical director of Pinnacle Clinical Research, and visiting professor of Hepatology, University of Oxford.
> Continue the interview
“We see a lot of patients with fatty liver disease in our clinics and conduct many clinical trials in this therapeutic area. From a clinical research perspective, FibroScan® helps characterize patients and ultimately improves the rate at which we find patients who will be eligible for participation in clinical trials. It has played an important role at our four clinical research centers, and we are very pleased with this non-invasive means of assessing liver health.”
Dr. Juan Pablo Frias |M.D., Medical Director and Principal Investigator, National Research Institute, Los Angeles, USA
Watch our webinar to learn how FibroScan® Solutions can support through all the steps of your trial journey. Professor Castera reviews the benefits of FibroScan® and its composite Scores in late-phase MASH trials, including showing the capability to significantly reduce screen failure rates, monitor patients, and accelerate confirmation as a clinical benefit.
On top of the NITs usage, the Echosens team presents how we partner with the clinical sites and sponsors to implement robust quality control processes to support standardization of FibroScan® data.