
Guided VCTE™
Seamless liver health assessment for all
The complete non-invasive solution for liver disease management.
Powered by LSM by VCTE™, CAP™ and SSM by VCTE™ as part of an overall assessment of the liver.
Ideal point-of-care solution for high-volume hospital-based settings.


The spleen stiffness measurement (SSM by VCTE™) enables risk stratification of patients with advanced chronic liver disease and portal hypertension.1, 2, 3
The embedded ultrasound guidance eases both liver and spleen targeting.
Assess and Monitor Portal Hypertension*
SSM by VCTE™ helps to assess and monitor portal hypertension, the main driver of cirrhosis, in a quick and non-invasive way.
Helps to Assess the Presence of Esophageal Varices*
SSM by VCTE™ can provide added value to help identify high-risk varices and prioritize endoscopies.
Determine Surgery*
SSM by VCTE™ has the potential to triage patients in the general surgical population by assessing portal hypertension, a known risk factor, prior to surgery.
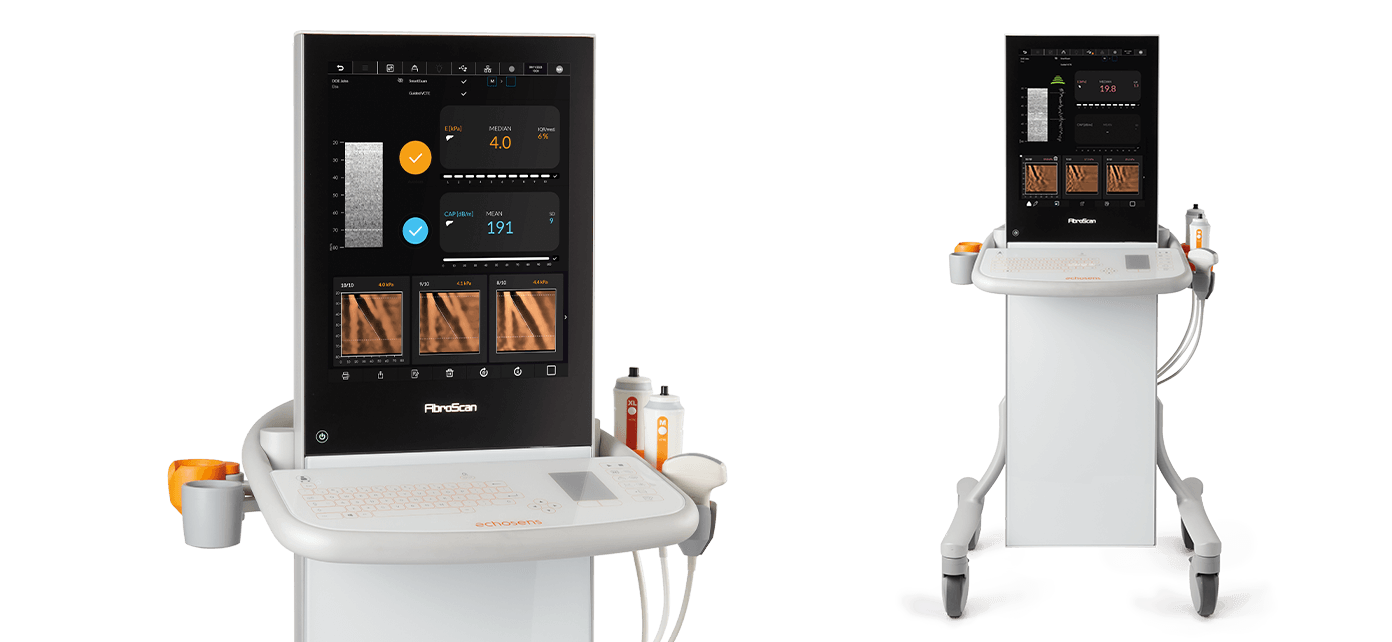
FibroScan® Expert 630 offers a high-speed processing, 19-inch touchscreen and a washable touch keyboard.
For the spleen examination with SSM by VCTE™, the M+ probe automatically adjusts to 100 Hz, adapts measurement depth, and adjusts the stiffness range. The ultrasound localization probe is a time-saving technology for easily locating the spleen and liver in complex patients and patients with obesity.
FibroScan® Expert 630 is adapted to all patient morphologies as it can be used with all probes S+, M+ and XL+. The two probe connectors allows to easily switch between probes during exam.
From installation to training and local support, we provide you with the highest quality of services.
*SSM is a marker for non-invasive evaluation of spleen stiffness which has been used in a clinical setting to assess portal hypertension and for variceal surveillance.
1.Stefanescu H, et al. A novel spleen-dedicated stiffness measurement by FibroScan improves the screening of high-risk oesophageal varices. Liver Int. 2020;40(1):175-185. doi:10.1111/liv.14228.
2. Dajti, Elton et al. “A Combined Baveno VII and Spleen Stiffness Algorithm to Improve the Noninvasive Diagnosis of Clinically Significant Portal Hypertension in Patients With Compensated Advanced Chronic Liver Disease.” The American journal of gastroenterology vol. 117,11 (2022): 1825-1833. doi:10.14309/ajg.0000000000001887.
3. Baveno VII – R. de Franchis et al. Renewing consensus in portal hypertension. Journal of hepatology vol. 76,4 (2022): 959-974. doi:10.1016/j.jhep.2021.12.022.
The FibroScan® Expert 630 is intended to measure liver stiffness (E) using Vibration Controlled Transient Elastography (VCTE™ ) at 50 Hz shear wave frequency and liver ultrasound attenuation coefficient (CAP™)* at 3.5 MHz. FibroScan® 630 Expert is also intended to measure spleen stiffness using VCTE™ at 100 Hz shear wave frequency. FibroScan liver stiffness measurements (LSM) by VCTE™ may aid the physician in determining the likelihood of cirrhosis and may be used, taken in context with other clinical and laboratory data, as an aid in the assessment of liver fibrosis. FibroScan CAP™ measurements may be used, taken in context with other clinical and laboratory data, as an aid in the assessment of hepatic steatosis. FibroScan® is indicated as a non-invasive aid to clinical management, diagnosis, and monitoring of adult and pediatric patients with confirmed or suspected liver disease, as part of an overall assessment of the liver. Results in the pediatric population should be interpreted while considering the clinical condition and the overall patient profile. The FibroScan® device is intended for use by healthcare professionals in hospitals, clinics or any facility where healthcare is provided.
*CAP™ refers to ultrasound attenuation coefficient (originally defined as Controlled Attenuation Parameter). CAP™ on S+ probe is only available with SmartExam capability.

Seamless liver health assessment for all

Optimize measurement accuracy on all patients of varying size
Optimize clinical workflows with real-time secure data transmission

Cloud-based solution to assist clinicians in providing comprehensive liver care.