
Liver Health Management
Cloud-based solution to assist clinicians in providing comprehensive liver care.
The state-of-the-art non-invasive solution for liver disease management, affordable to all.
Powered by LSM by VCTE™ and CAP™ for liver fibrosis and steatosis management.
Supported by 5,384+ peer reviewed publications and 218+ guidelines.
Ideal point-of-care solution for primary care and endocrinology in office-based settings.


Comprehensive and flexible pay-per-exam offer.
All services included.
Screening of at-risk patients for early intervention.
Simple, quick, non-invasive and painless examination.
Interpretation tools and calculation of clinical scores
to support in making instant medical decisions for patients.
Assists in monitoring staged progression of liver disease
which may lead to improved patient outcomes.
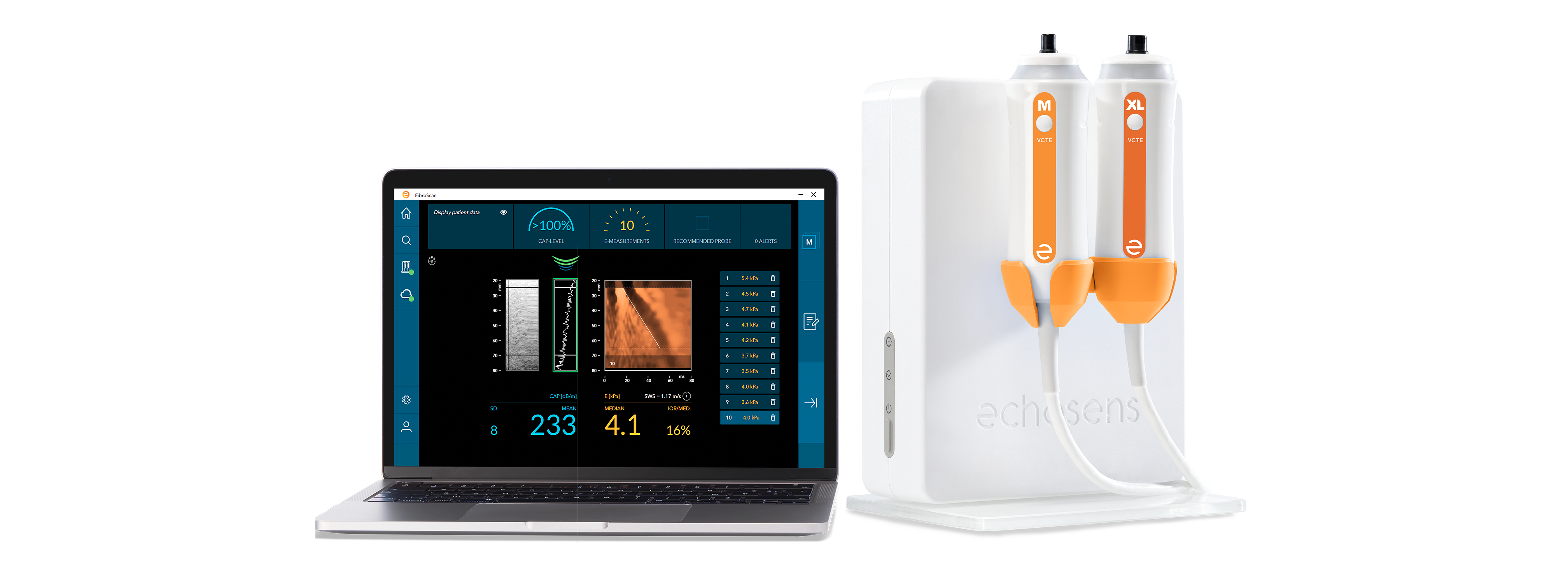
Perform the examination with FibroScan® and interpretation
to determine the fibrosis stage and the steatosis grade.
Calculate FIB-4 and combined scores.
Generate consolidated and personalized medical reports.
Consult and visualize the history of your patients’ results.
Assists with onsite installation and training.
Includes maintenance, annual calibration of probes and both software upgrades and updates.
Easy access to continuous training and users community with Echosens Academy.
Ideal point-of-care solution for primary care and endocrinology in office-based settings.
Equipment compatible with an S+ probe (sold separately).
The FibroScan® 230 is intended to measure liver stiffness (E) using Vibration Controlled Transient Elastography (VCTE™) at 50 Hz shear wave frequency and liver ultrasound attenuation (CAP™)* at 3.5 MHz. FibroScan liver stiffness measurements (LSM) by VCTE™ may aid the physician in determining the likelihood of cirrhosis and may be used, taken in context with other clinical and laboratory data, as an aid in the assessment of liver fibrosis. FibroScan CAP™ measurements may be used, taken in context with other clinical and laboratory data, as an aid in the assessment of hepatic steatosis. FibroScan® is indicated as a non-invasive aid to clinical management diagnosis and monitoring of adult and pediatric patients with confirmed or suspected liver disease, as part of an overall assessment of the liver. Results in the pediatric population should be interpreted while considering the clinical condition and the overall patient profile. The FibroScan® device is intended for use by healthcare professionals in hospitals, clinics or any facility where healthcare is provided.
*CAP™ refers to ultrasound attenuation coefficient (originally defined as Controlled Attenuation Parameter).
All personal and health data generated by the FibroScan® GO solution is managed in compliance with HIPAA regulations. Data located on Echosens Cloud is managed by an ISO27001 certified organization.
1. Wong VW, et al. Noninvasive biomarkers in NAFLD and NASH – current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15(8):461-478.
2. European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237-264.
3. Steadman R, et al. A health technology assessment of transient elastography in adult liver disease. Can J Gastroenterol. 2013;27(3):149-158.
To access the FibroScan® 230 user guides, connect to the Echosens Cloud
Learning Center > FibroScan® GO

Cloud-based solution to assist clinicians in providing comprehensive liver care.

Optimize measurement accuracy on all patients of varying size

The non-invasive solution for comprehensive management of liver health
