FibroScan® procedure for users
Find the key recommendations to run a liver examination with FibroScan®

Pre examination requirements
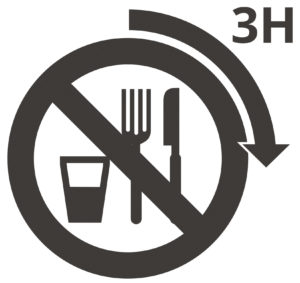
Patient shall be fasting for at least 3 hours-however, they can drink water prior to their scan.1,2,3
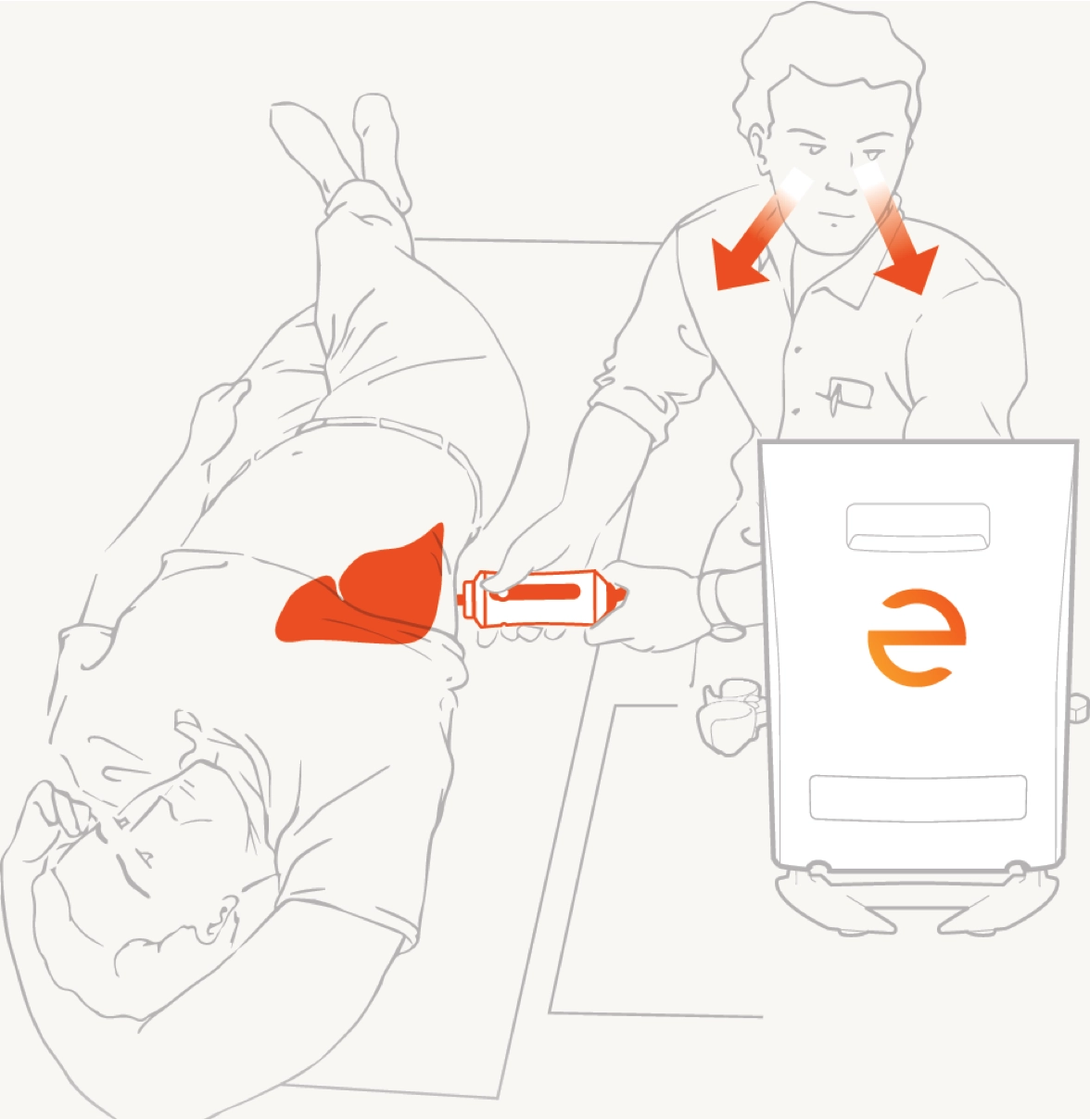
Patient shall be lying on the examination bed for 5 minutes before the examination for the body to rest.
Operator shall be duly trained and certified by Echosens or its local representative.
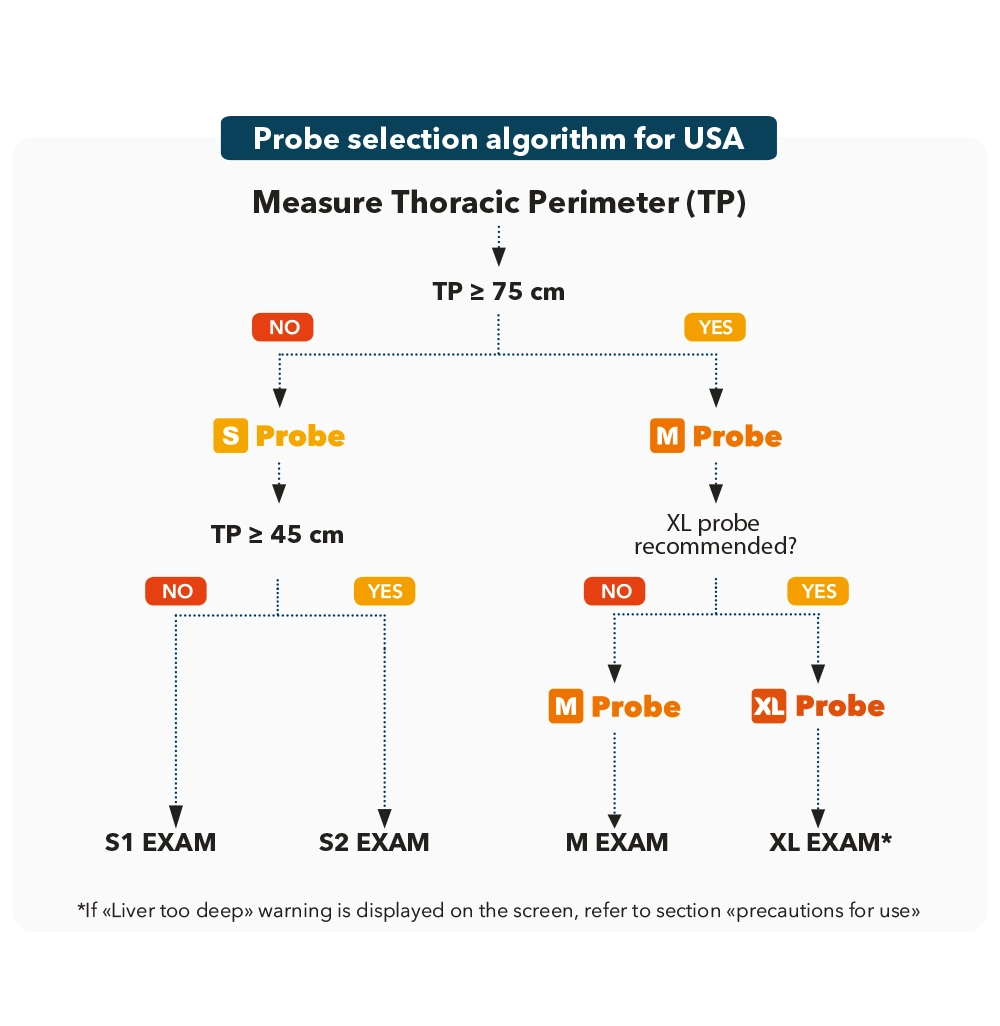
Device probes shall be calibrated.
Watch the liver examination with FibroScan® tutorial
Find out more exclusive tips & tutorials on echosensacademy.com to improve your FibroScan® daily practice.
To improve your FibroScan® skills, review the reminder sheets and tips available on echosensacademy.com.
No, FibroScan® can be performed on every single patient. Examinations on pregnant women and patients with active implantable medical devices (pacemaker, defibrillator…) are not contraindicated.
Contraindications have been lifted since April 2016 in Europe, and subsequently in all other countries (including the United States since March 2023), except in Japan.
References on Pacemakers and Defibrillators
1/ Chan Y, et al. Safety Profile of Liver FibroScan in Patients with Cardiac Pacemakers or Implantable Cardioverter-Defibrillators. Can J Gastroenterol Hepatol. 2017;2017:7298032. doi: 10.1155/2017/7298032. Epub 2017 Feb 27. PMID: 28349045; PMCID: PMC5350419.
2 / Friedrich-Rust M, et al. Safety of transient elastography in patients with implanted cardiac rhythm devices. Dig Liver Dis. 2017 Mar;49(3):314-316. doi: 10.1016/j.dld.2016.11.005. Epub 2016 Nov 17. PMID: 27908579.
References on Pregnant Patients
1/ Duvekot J, et al. [5-OR]: Transient elastography (TE) of the liver as a new diagnostic tool to discriminate between HELLP syndrome and acute fatty liver of pregnancy (AFLP)?, Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health, Volume 5, Issue 1,
2015, Pages 2-3, ISSN 2210-7789, https://doi.org/10.1016/j.preghy.2014.10.009.
2/ Liver stiffness reversibly increases during pregnancy and independently predicts preeclampsia
Ammon FJ, et al. Liver stiffness reversibly increases during pregnancy and independently predicts preeclampsia. World J Gastroenterol. 2018 Oct 14;24(38):4393-4402. doi: 10.3748/wjg.v24.i38.4393. PMID: 30344423; PMCID: PMC6189842.
Probes are calibrated by Echosens support. All you need to know about probe calibration is available in the calibration FAQ.
The Interpretation Guide and cut-offs are on myFibroScan (app and website).
Peer-reviewed articles used for the cut-off are available in our Clinical Library.
Training and certification by Echosens or its local representatives is prerequisite for every FibroScan® user.
Connect with your local representative or contact us directly through the contact page available on this website.
There are many clinical studies available for reference in our clinical library.
– For methotrexate, filter your search by selecting Etiology = “Drug induced liver injury (DILI)”.
– For primary biliary cirrhosis and primary sclerosing cholangitis, filter your search by selecting Etiology = “Cholestatic disease”.
– For haemochromatosis, filter your search by selecting aetiology = “Other”.
The present page is not intended to provide training or certification to users.
1. Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, Bahr MJ. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver International 2009;29:1500-1506.
2. Arena U, Lupsor Platon M, Stasi C, Moscarella S, Assarat A, Bedogni G, Piazzolla V, et al. Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology 2013;58:65-72.
3. Berzigotti A, De Gottardi A, Vukotic R, Siramolpiwat S, Abraldes JG, Garcia-Pagan JC, Bosch J. Effect of meal ingestion on liver stiffness in patients with cirrhosis and portal hypertension. PLoS One 2013;8:e58742.
The FibroScan® Family of Products (models: Compact 530, Mini+ 430 and Expert 630) is intended to provide 50Hz shear wave speed measurements and estimates of tissue stiffness as well as 3.5 MHz ultrasound coefficient of attenuation (CAP™: Controlled Attenuation Parameter) in internal structures of the body. The shear wave speed and stiffness, and CAP™ may be used as an aid to diagnosis and monitoring of adult patients with liver disease, as part of an overall assessment of the liver. Shear wave speed and stiffness may be used as an aid to clinical management of pediatric patients with liver disease. The FibroScan® 630 (Expert) is also indicated for noninvasive measurement in the spleen of 100 Hz shear wave speed and estimates of stiffness that may be used as an aid to diagnosis, monitoring and clinical management of adult patients with liver disease, as part of an overall assessment of the liver.