
Guided VCTE™
Seamless liver health assessment for all
The mobile non-invasive solution for liver disease management, ideal for multi-site configuration.
Powered by LSM by VCTE™ and CAP™ for liver disease management.
Ideal point-of-care solution for large sized and community hospital-based settings.


FibroScan® Mini+ 430 is designed for multi-center sharing and remote liver patient management.
FibroScan® Mini+ 430 is light and easy to handle (11 lbs.). It is a battery-powered device with a 12.1-inch touchscreen.
Winner of the Red Dot Design Award 2017 for its innovative and ergonomic design, FibroScan® Mini+ 430 is provided with airplane-compatible transport suitcase.
FibroScan® Mini+ 430 is adapted to all patient morphologies as it can be used with all probes S+, M+ and XL+. The two probe connectors allows to easily switch between probes during exam.
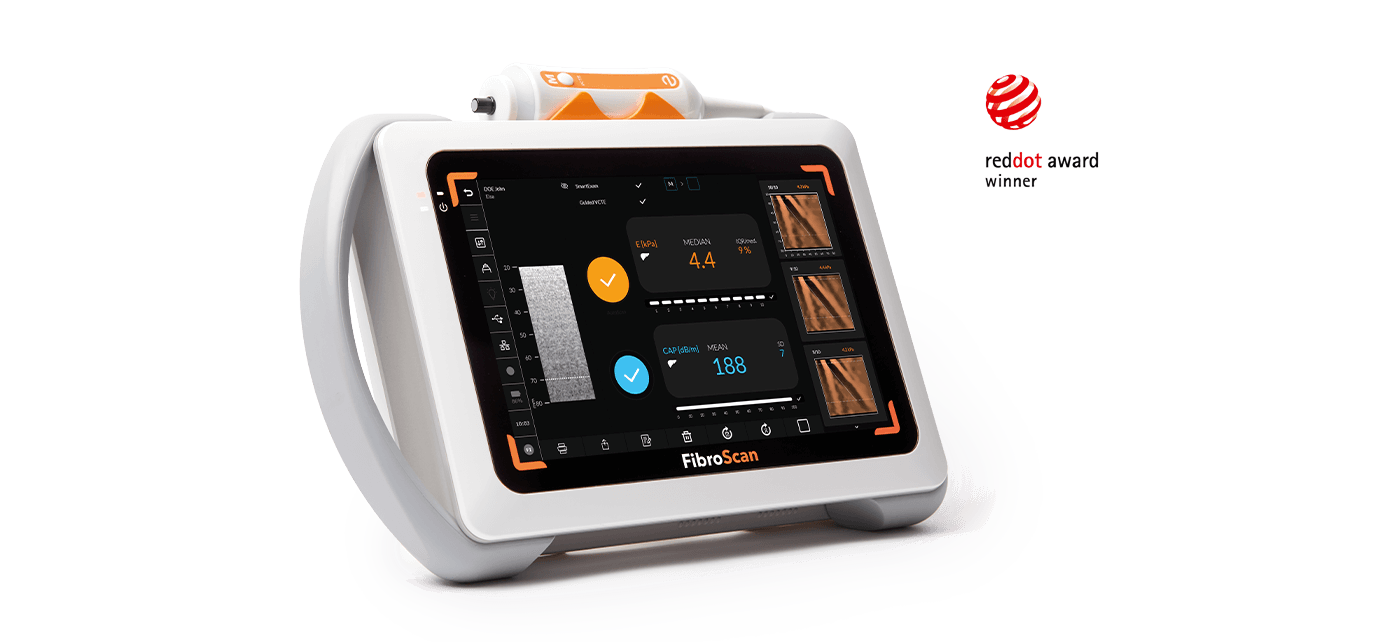
LSM by VCTE™ is unique, patented and validated for liver fibrosis assessment. It is the standard for non-invasive evaluation of liver stiffness with 4,778+ international peer-reviewed publications.1
CAP™ is unique patented and validated for liver steatosis assessment2,3 with 1,653+ international peer-reviewed publications.
From installation, to training and local support, we provide you with the highest quality of services.
1. European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237-264. doi:10.1016/j.jhep.2015.04.006.
2. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022-1030. doi:10.1016/j.jhep.2016.12.022.
3. Recio E, Cifuentes C, Macías J, et al. Interobserver concordance in controlled attenuation parameter measurement, a novel tool or the assessment of hepatic steatosis on the basis of transient elastography. Eur J Gastroenterol Hepatol. 2013;25(8):905-911.doi:10.1097/MEG.0b013e32835f4c3d.
The FibroScan® 430 Mini+ is intended to measure liver stiffness (E) using Vibration Controlled Transient Elastography (VCTE™) at 50 Hz shear wave frequency and liver ultrasound attenuation coefficient (CAP™)* at 3.5 MHz. FibroScan® liver stiffness measurements (LSM) by VCTE™ may aid the physician in determining the likelihood of cirrhosis and may be used, taken in context with other clinical and laboratory data, as an aid in the assessment of liver fibrosis. FibroScan® CAP™ measurements may be used, taken in context with other clinical and laboratory data, as an aid in the assessment of hepatic steatosis. FibroScan® is indicated as a non-invasive aid to clinical management, diagnosis, and monitoring of adult and pediatric patients with confirmed or suspected liver disease, as part of an overall assessment of the liver. Results in the pediatric population should be interpreted while considering the clinical condition and the overall patient profile. The FibroScan® device is intended for use by healthcare professionals in hospitals, clinics or any facility where healthcare is provided. * CAP™ refers to ultrasound attenuation coefficient (originally defined as Controlled Attenuation Parameter). CAP™ on S+ probe is only available with SmartExam capability.

Seamless liver health assessment for all

Optimize measurement accuracy on all patients of varying size
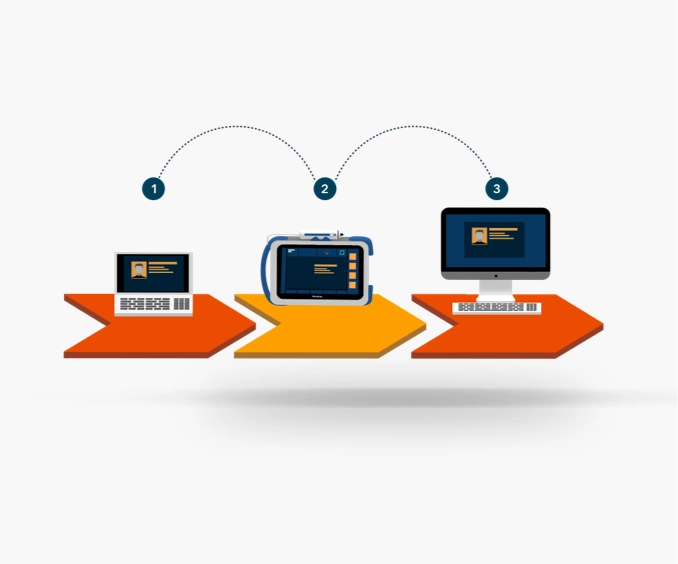
Optimize clinical workflows with real-time secure data transmission

Cloud-based solution to assist clinicians in providing comprehensive liver care.