FibroScan® procedure for users
Find the key recommendations to run a liver examination with FibroScan®

Pre examination requirements
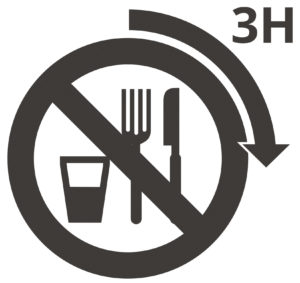
Patient shall be fasting for at least 3 hours and can take clear fluids only.1,2,3
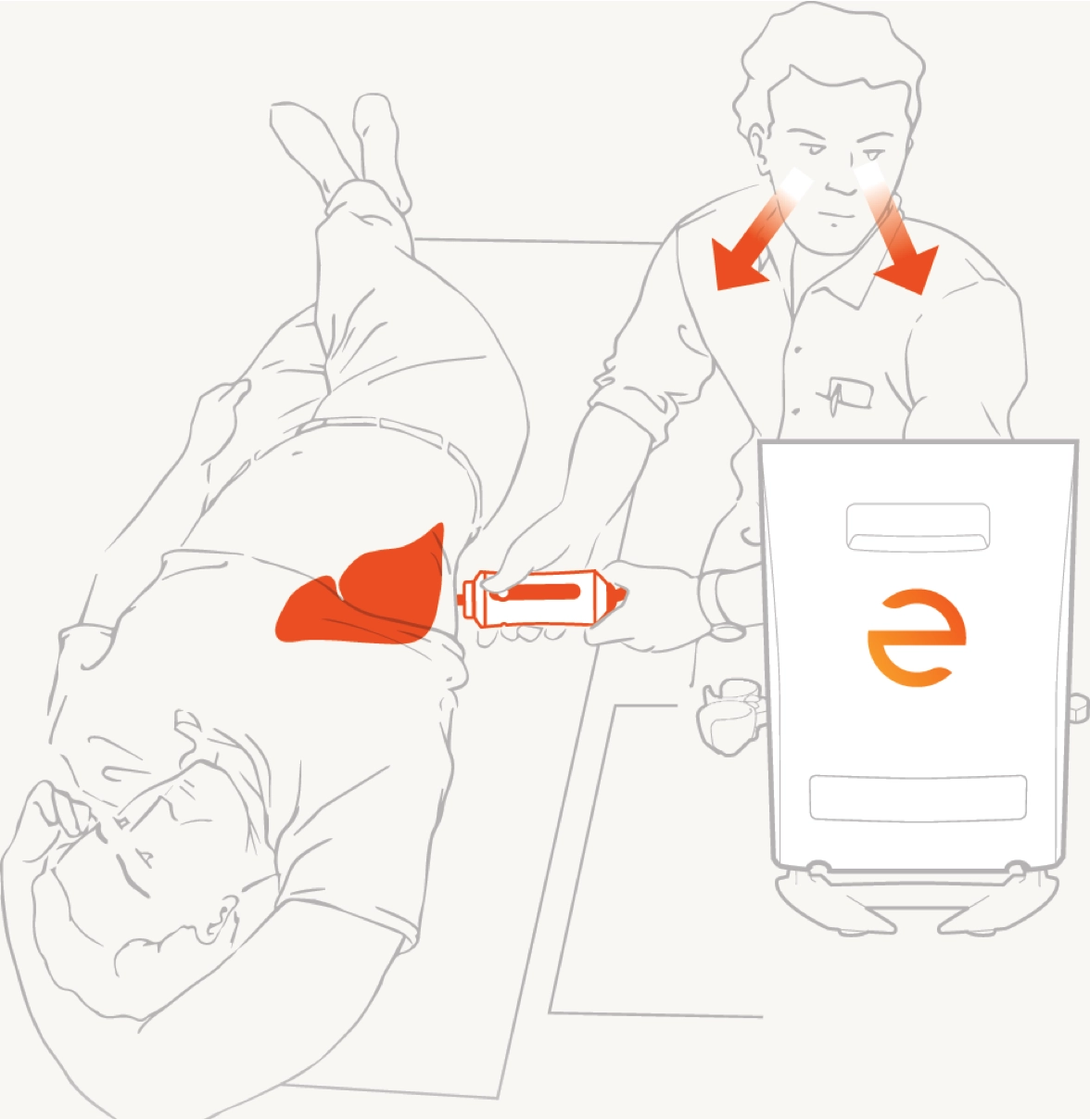
Patient shall be lying on the examination bed for 5 minutes before the examination for the body to rest.
Operator shall be duly trained and certified by Echosens or its local representative.
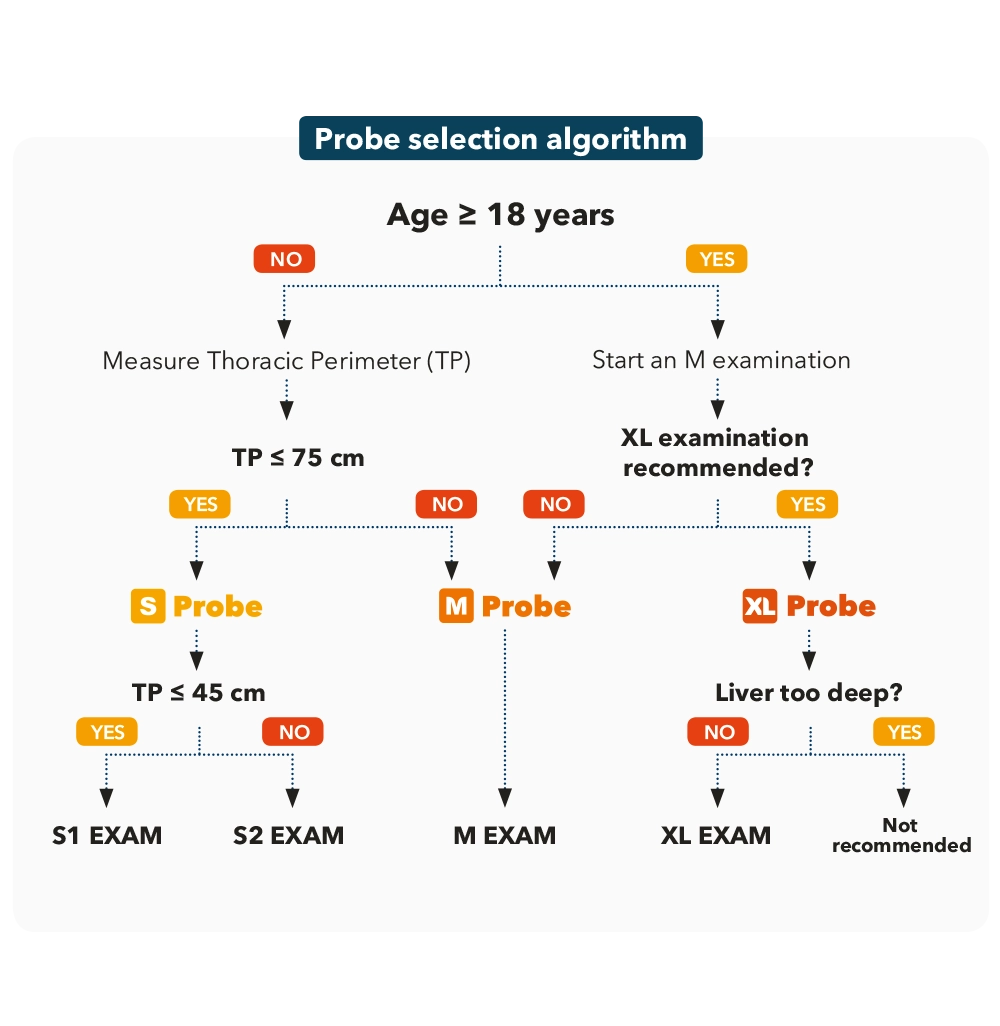
Device probes shall be calibrated.
Watch the liver examination with FibroScan® tutorial
Find out more exclusive tips & tutorials on echosensacademy.com to improve your FibroScan® daily practice.
To improve your FibroScan® skills, review the reminder sheets and tips available on echosensacademy.com.
No, FibroScan® can be performed on every single patient. Examinations on pregnant women and patients with active implantable medical devices (pacemaker, defibrillator…) are not contraindicated.
Contraindications have been lifted since April 2016 in Europe, and subsequently in all other countries (including the United States since March 2023), except in Japan.
References on Pacemakers and Defibrillators
1/ Chan Y, et al. Safety Profile of Liver FibroScan in Patients with Cardiac Pacemakers or Implantable Cardioverter-Defibrillators. Can J Gastroenterol Hepatol. 2017;2017:7298032. doi: 10.1155/2017/7298032. Epub 2017 Feb 27. PMID: 28349045; PMCID: PMC5350419.
2 / Friedrich-Rust M, et al. Safety of transient elastography in patients with implanted cardiac rhythm devices. Dig Liver Dis. 2017 Mar;49(3):314-316. doi: 10.1016/j.dld.2016.11.005. Epub 2016 Nov 17. PMID: 27908579.
References on Pregnant Patients
1/ Duvekot J, et al. [5-OR]: Transient elastography (TE) of the liver as a new diagnostic tool to discriminate between HELLP syndrome and acute fatty liver of pregnancy (AFLP)?, Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health, Volume 5, Issue 1,
2015, Pages 2-3, ISSN 2210-7789, https://doi.org/10.1016/j.preghy.2014.10.009.
2/ Liver stiffness reversibly increases during pregnancy and independently predicts preeclampsia
Ammon FJ, et al. Liver stiffness reversibly increases during pregnancy and independently predicts preeclampsia. World J Gastroenterol. 2018 Oct 14;24(38):4393-4402. doi: 10.3748/wjg.v24.i38.4393. PMID: 30344423; PMCID: PMC6189842.
Probes are calibrated by Echosens support. All you need to know about probe calibration is available in the calibration FAQ.
The Interpretation Guide and cut-offs are on myFibroScan (app and website).
Peer-reviewed articles used for the cut-off are available in our Clinical Library.
Training and certification by Echosens or its local representatives is prerequisite for every FibroScan® user.
Connect with your local representative or contact us directly through the contact page available on this website.
There are many clinical studies available for reference in our clinical library.
- For methotrexate, filter your search by selecting Etiology = “Drug induced liver injury (DILI)”.
- For primary biliary cirrhosis and primary sclerosing cholangitis, filter your search by selecting Etiology = “Cholestatic disease”.
- For haemochromatosis, filter your search by selecting aetiology = “Other”.
The present page is not intended to provide training or certification to users.
1. Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, Bahr MJ. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver International 2009;29:1500-1506.
2. Arena U, Lupsor Platon M, Stasi C, Moscarella S, Assarat A, Bedogni G, Piazzolla V, et al. Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology 2013;58:65-72.
3. Berzigotti A, De Gottardi A, Vukotic R, Siramolpiwat S, Abraldes JG, Garcia-Pagan JC, Bosch J. Effect of meal ingestion on liver stiffness in patients with cirrhosis and portal hypertension. PLoS One 2013;8:e58742.
4. Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, Fouchard-Hubert I, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013;57:1182-1191.
Products in the FibroScan® range are class IIa medical devices according to Rule 10 of ANNEX VIII of Regulation EU 2017/745 (CE 0459) and are manufactured by Echosens™. FibroScan® devices are intended to provide: Liver stiffness measurements at a shear wave frequency of 50 Hz, liver ultrasound attenuation measurements (CAP: Controlled Attenuation Parameter) at 3.5 MHz and spleen stiffness measurements at a shear wave frequency of 100 Hz (FibroScan® 630 Expert only). These non-invasive devices are intended to aid clinical management, diagnosis, and monitoring of patients with confirmed or suspected chronic liver disease, as part of an overall assessment of the liver. FibroScan® devices may aid the healthcare professionals in the assessment of liver fibrosis, steatosis, and in determining the likelihood of cirrhosis, and its complications (FibroScan® 630 Expert only). They are used, in conjunction with other clinical and laboratory data, during liver assessment in patients with confirmed or suspected chronic liver disease. Examinations with FibroScan® devices shall be performed by an operator who has been certified by the manufacturer or its approved local representative. Operators are expressly recommended to carefully read the instructions given in the user manual and on the labelling of these products. Check cost defrayal conditions with paying bodies.