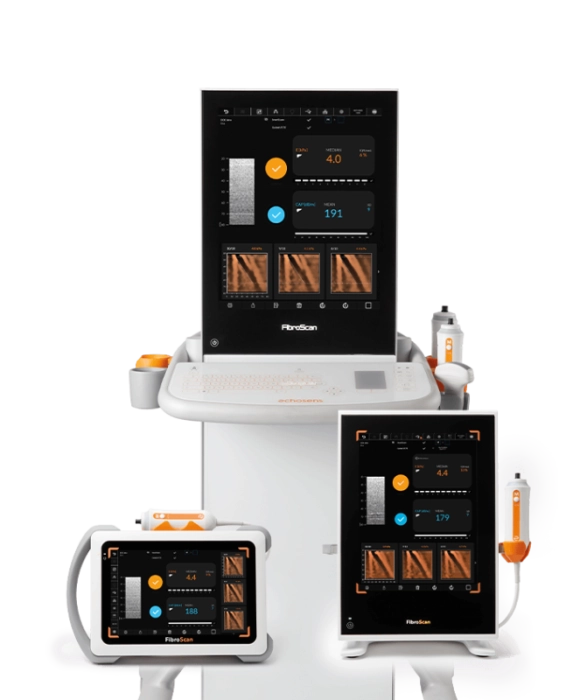
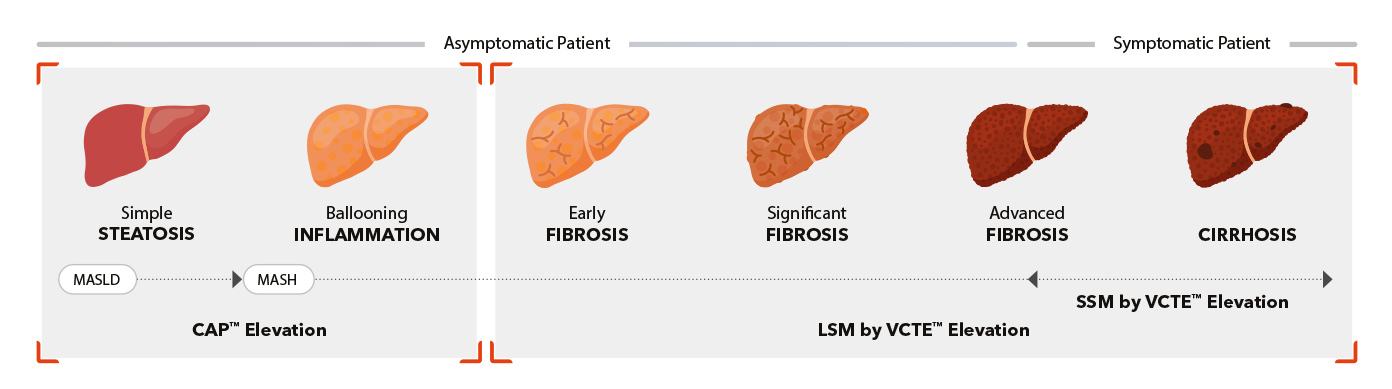
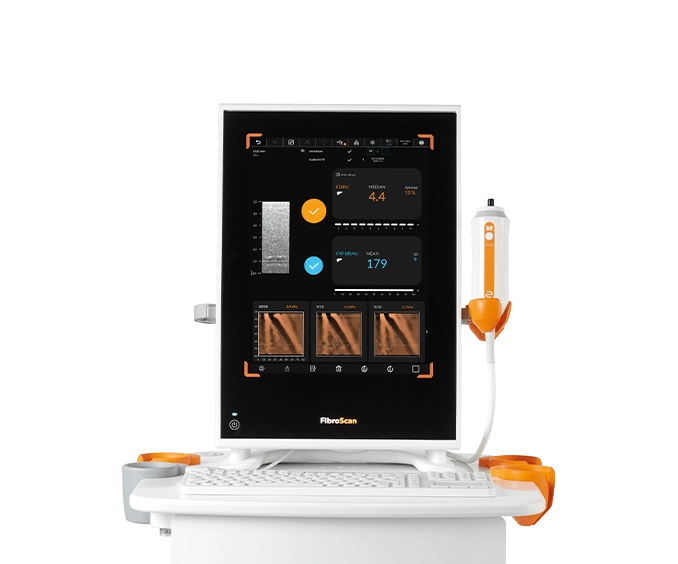
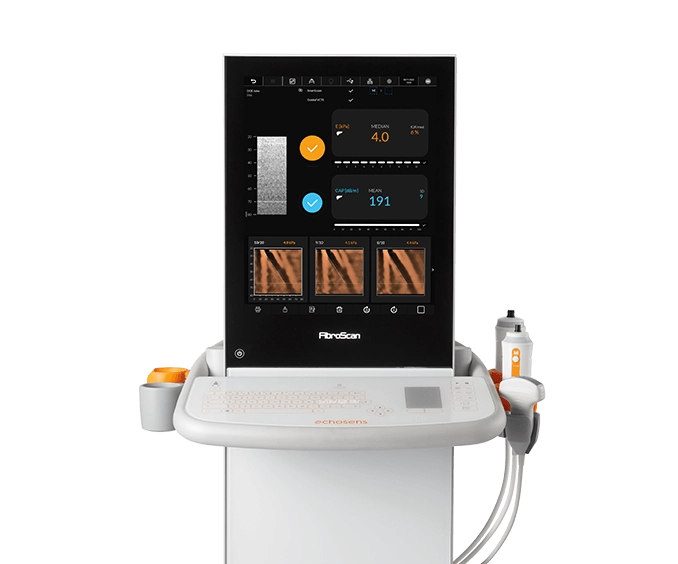
FibroScan® Mini+ 430
Capabilities
- Guided VCTE™
- LSM by VCTE™
- CAP™
Features
- LHM compatibility
- FibroScan® Gateway compatibility
- myFibroScan compatibility
- Two probe connectors
Ergonomics
- Fully transportable
- Battery-powered
- Airplane-compatible suitcase
- Weight : 5kg