FibroScan® Solutions in MASH* Clinical Trials
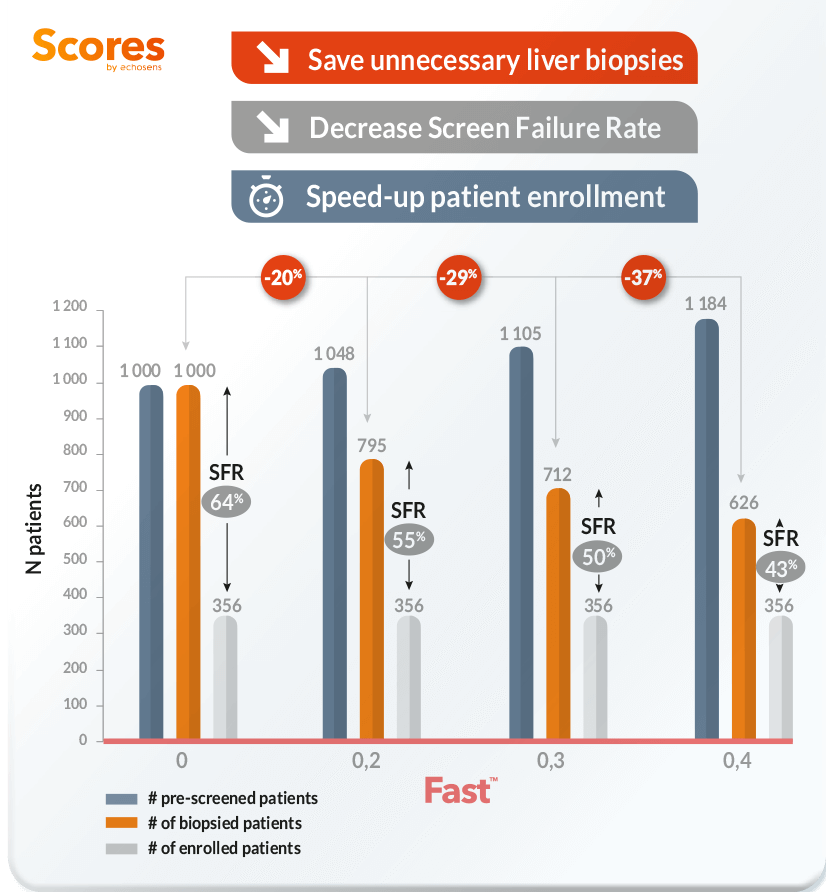
FibroScan® and its associated solutions can be leveraged to significantly reduce screen failure rate, accelerate patient enrollment, and monitor therapeutic response.
Download the White Paper and enjoy the reading!
*Formerly known as NASH

Our solutions can support the development of MASH therapies
with the reference liver non-invasive point-of-care testing solutions
- Accelerating drug development,
- De-risking trial execution
- Preparing for commercialization
Overview of the white paper
Schematic of a typical Phase 3/4 clinical trial for accelerated drug approval
to treat patients with non-cirrhotic MASH.
(EOT: End of Treatment, M: Month)
Impact of the use of Fast™ on patient enrollement
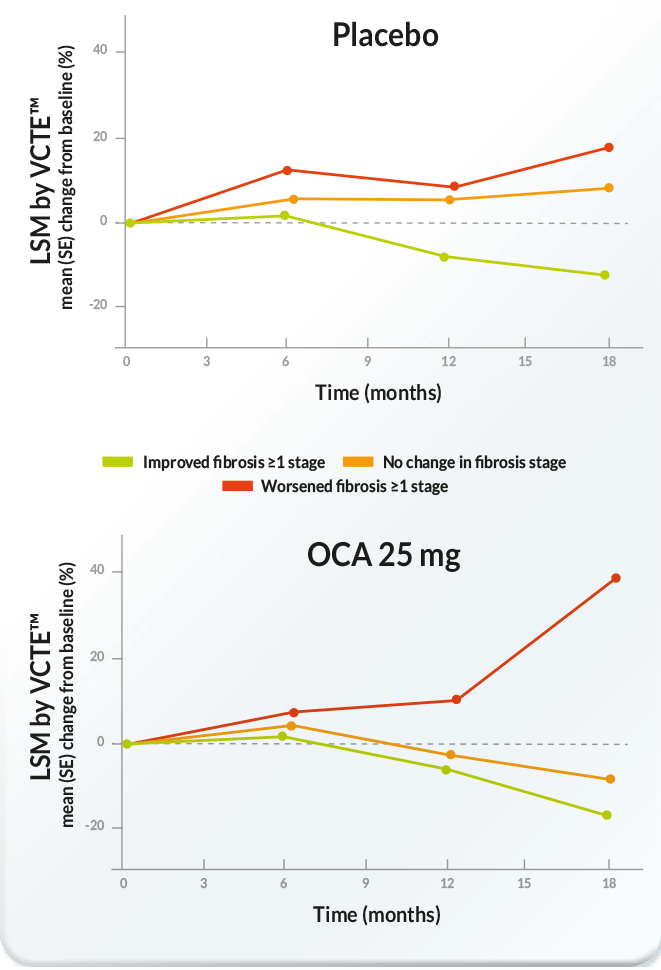
Mean percentage change from baseline in LSM by VCTE™ over time
by treatment group and histological fibrosis improvement status
(adapted from [1])
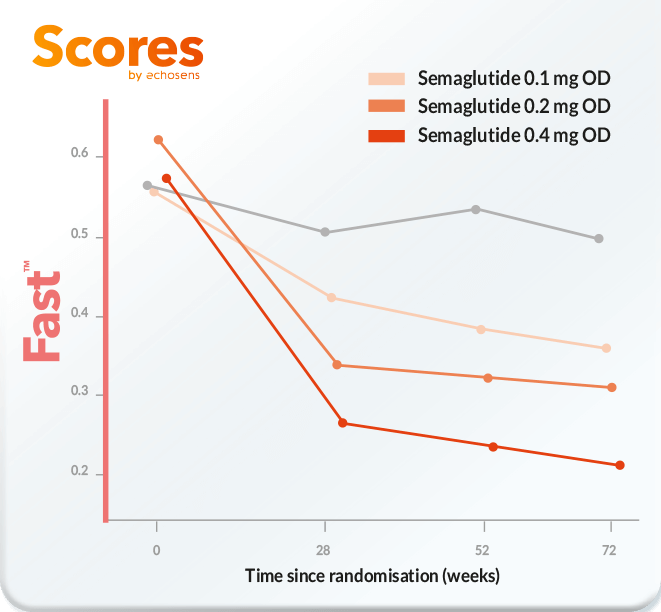
Change from baseline to week 72 of Fast™ score
Subcutaneous Semaglutide Phase 2b trial
(adapted from [2])
[1] – Rinella M, Dufour J-F, Anstee QM, Goodman Z, Younossi Z, Harrison SA et al. Non-invasive evaluation of response to obeticholic acid in patients with MASH: Results from the REGENERATE study. Journal of Hepatology 2021.
[2] – Wong VW-S, Quentin A, Geerts A, Mette K, Ladelund S, Ratziu V et al. Change in FibroScan-aspartate aminotransferase (FAST) score is associated with histological improvement in non-alcoholic steatohepatitis activity: The Journal of Hepatology; 2021.
Products in the FibroScan® range are class IIa medical devices according to Directive 93/42/EEC and are manufactured by Echosens™. CE 0459 – ISO 13485. This device is designed to be used in a physician’s office to measure the stiffness and ultrasonic attenuation of the liver in patients with liver disease. It is expressly recommended to carefully read the guidance and instruction of the users’ guide and labeling of the device. Results obtained must be interpreted by a physician experienced in dealing with liver disease, taking into account the complete medical record of the patients.