
Liver Health Management
Cloud-based solution to assist clinicians in providing comprehensive liver care.
The state-of-the-art non-invasive solution, affordable to all.
Powered by LSM by VCTE™ and CAP™ for liver fibrosis and steatosis management.
Supported by 5,384+ peer reviewed publications and 218+ guidelines.


Pay per exam offer.
All services included.
Early detection of patients at risk.
Simple, quick, non-invasive and painless examination
that can be performed by any trained operator.
Interpretation tools and calculation of clinical scores
to support in making instant medical decisions for patients.
Diagnosis and follow-up of the progression of the disease.
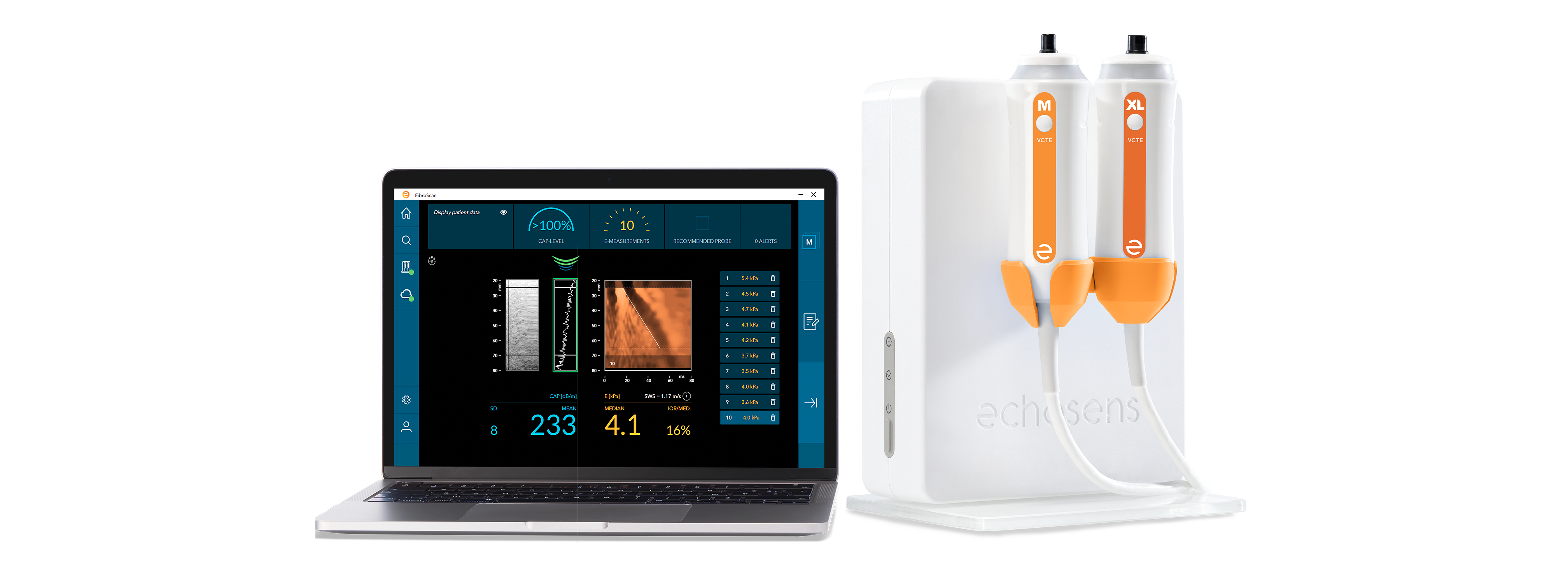
Perform the examination with FibroScan® and its interpretation
to determine the fibrosis stage and the steatosis grade.
Calculate FIB-4 and Combined Scores.
Generate consolidated and personalized medical reports.
Consult and visualize the history of your patients’ results.
Assists you with onsite installation and training.
Includes maintenance, calibration of probes and software updates.
Easy access to continuous training and users community with Echosens Academy.
FibroScan® 230 is a class IIa medical devices according to Rule 10 of ANNEX VIII of Regulation EU 2017/745 (CE 0459) and is manufactured by Echosens™. FibroScan® 230 is intended to provide Liver stiffness measurements at a shear wave frequency of 50 Hz and Liver ultrasound attenuation measurements (CAP: Controlled Attenuation Parameter) at 3.5 MHz. FibroScan® 230 is a non-invasive device intended to aid clinical management, diagnosis, and monitoring of patients with confirmed or suspected chronic liver disease, as part of an overall assessment of the liver. FibroScan® device may aid the healthcare professionals in the assessment of liver fibrosis, steatosis, and in determining the likelihood of cirrhosis.
FibroScan® device is used, in conjunction with other clinical and laboratory data, during liver assessment in patients with confirmed or suspected chronic liver disease. Examinations with FibroScan® device shall be performed by an operator who has been certified by the manufacturer or its approved local representative. Operators are expressly recommended to carefully read the instructions given in the user manual and on the labelling of these products. Check cost defrayal conditions with paying bodies.
All personal and health data generated by the FibroScan® GO solution is managed in compliance with local data privacy and GDPR regulations. Data located on Echosens Cloud is managed by an ISO 27001 certified organization.
1. Wong VW, et al. Noninvasive biomarkers in NAFLD and NASH – current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15(8):461-478.
2. European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237-264.
3. Steadman R, et al. A health technology assessment of transient elastography in adult liver disease. Can J Gastroenterol. 2013;27(3):149-158.
To access the FibroScan® 230 user guides, connect to the Echosens Cloud
Learning Center > FibroScan® GO

Cloud-based solution to assist clinicians in providing comprehensive liver care.

Optimize measurement accuracy on all patient morphologies

The non-invasive gold standard solution for comprehensive management of liver health
