
FibroScan® by echosens
The non-invasive gold standard solution for comprehensive management of liver health
Optimize measurement accuracy on all patient morphologies
Choose the appropriated probe between S+, M+ or XL+.

FibroScan® offers a range of 3 probes (S+, M+ and XL+) designed to fit patient morphology.
By adjusting the measurement area relative to the distance between the probe and the liver (Probe to Capsula Distance) a consistent explored volume can be maintained.

|
S+ probe |
M+ probe |
XL+ probe |
|
For pediatric population Designed for being placed into narrow intercostal space |
For patients
|
For patients with obesity Designed to enhance signal penetration |
|
Measurement depth From 15 to 50 mm |
Measurement depth From 25 to 70 mm |
Measurement depth From 35 to 85 mm |
|
Criteria of selection Age < 18yo
|
Criteria of selection Age < 18yo or Age ≥ 18yo |
Criteria of selection Age ≥ 18ans et |
For M+ and XL+ probes, FibroScan® automatically recommends the right probe.
Probes selection criteria
To ensure that the probe is adapted to the patient’s morphology, please read the FibroScan® examination procedure.
FibroScan® comes with the standard M+ probe.
S+ and XL+ probes are available as an option.
Products in the FibroScan® range are Class IIa medical devices as defined by Directive 93/42/EEC (EC 0459). These devices are designed for use in a medical practice in order to measure liver stiffness and ultrasound attenuation in patients with liver disease. Examinations with FibroScan® device shall be performed by an operator who has been certified by the manufacturer or its approved local representative. Operators are expressly recommended to carefully read the instructions given in the user manual and on the labelling of these products. Check cost defrayal conditions with paying bodies.

The non-invasive gold standard solution for comprehensive management of liver health
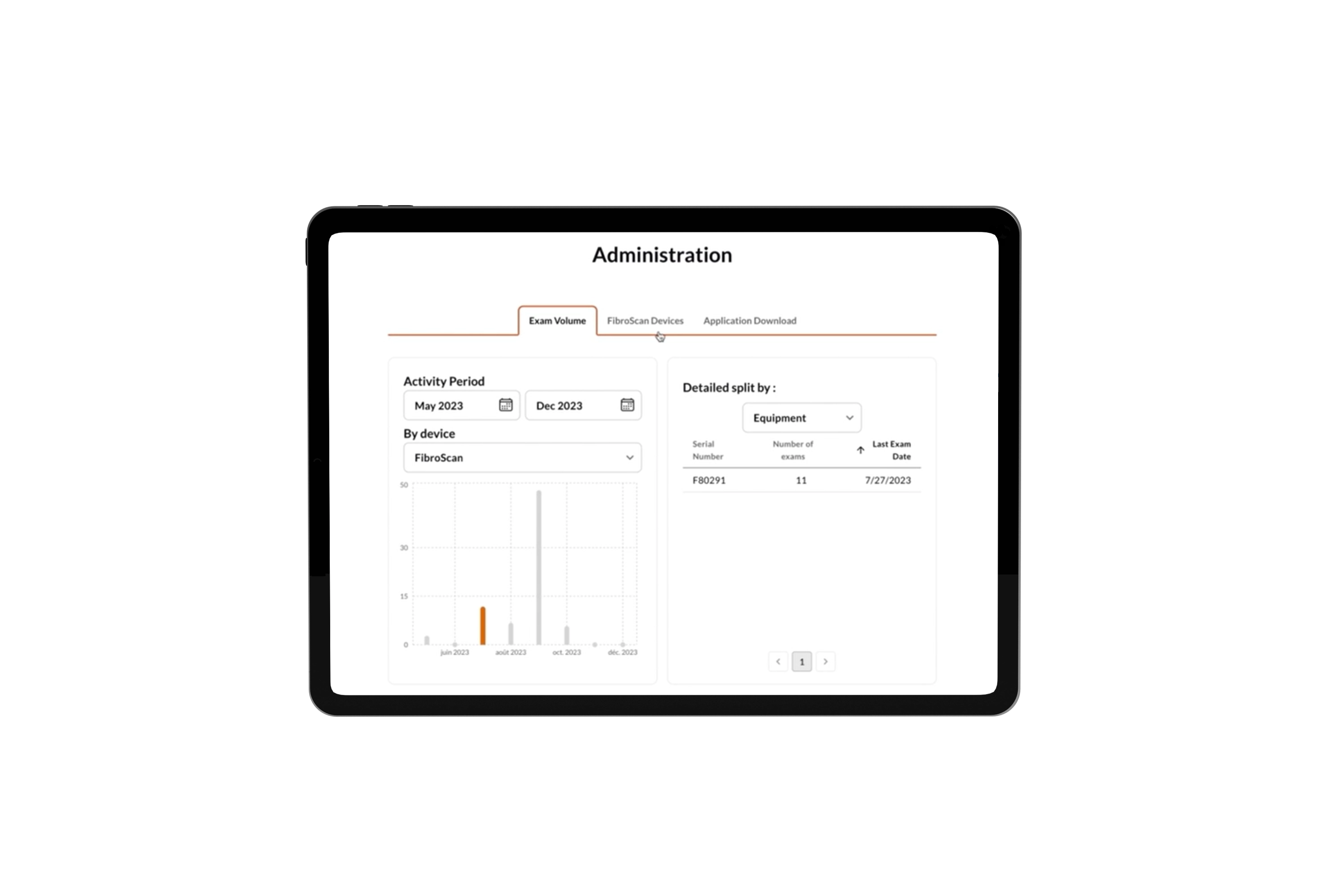
Cloud-based solution to assist clinicians in providing comprehensive liver care.

Enhance FibroScan® liver disease assessment with biological markers
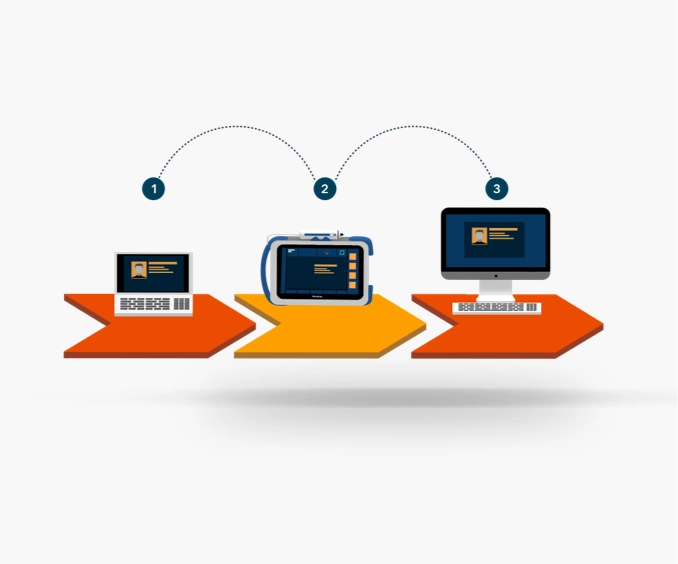
Optimize clinical workflows with real-time secure data transmission