
FibroScan® by echosens
The non-invasive gold standard solution for comprehensive management of liver health
Seamless liver health assessment for all
As a leader in liver health, we have elevated our core liver elastography technology to the next level with the introduction of Guided VCTE™ – our Next-Generation FibroScan® technology.


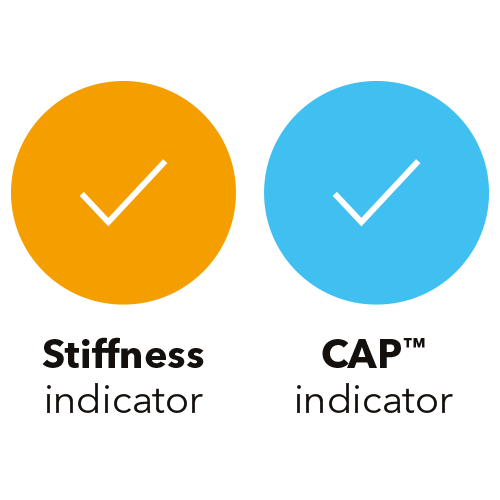
Two new visual indicators allow operators to quickly identify
the optimal measurement location.1
Computed by a continuous vibration of the probe transducer,
these indicators intuitively guide the operator throughout the entire
duration of the exam.
With Guided VCTE™, 96% of patients can be scanned in less than 4 minutes,
including patients with BMI≥35.1
Based on the Guided VCTE™ indicators, AutoScan automatically triggers
10 valid measurements through a single click, enabling streamlined scanning
and a likely improved operator experience.
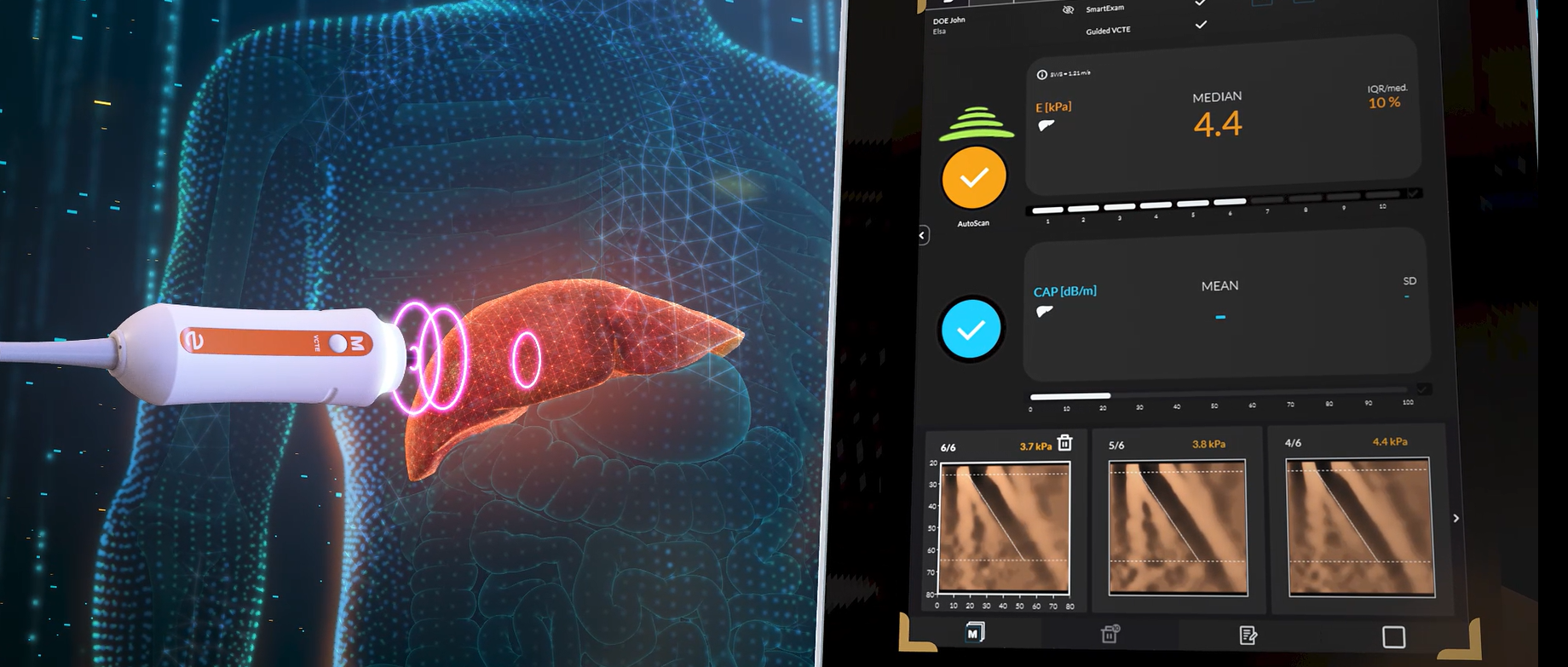
Improved reliability in the diagnosis and monitoring of steatosis
with Continuous CAP™ to decrease variability by 50%.2
Extended usage among patients with severe obesity with deeper
assessment of liver fibrosis and steatosis as the maximum probe-to-capsula
distance is increased by 28%*.
Automated features such as CAP™ validity criteria and automatic measurement
depth selection to adapt to patient’s morphology.
In this webinar, hear from key leaders in liver health detail their experience using the next-generation FibroScan® system, equipped with new Guided VCTE™ technology.
Learn more about how this technology impacts both the operator and physician experience, with a focus on optimizing liver health management through efficiency and standardization.
This white paper provides a technology overview of Guided VCTE™
and its associated benefits.
From installation, to training and local support, we provide you with the highest quality of services.
* Without SmartExam, the maximum recommended Probe-to-Capsula distance (PCD) is 35 mm. With SmartExam, it is 45mm, representing an increase of 28%.
References
1. Bastard, Cécile et al. “Guided-VCTE: An Enhanced FibroScan Examination With Improved Guidance and Applicability.” Ultrasound in medicine & biology vol. 51,4 (2025): 628-637.
2. E. Bardou-Jacquet et al. Validation of the Continuous Controlled Attenuation Parameter (CAPc) using the MRI-PDFF as reference. EASL 2022 poster #FRI-228.
Products in the FibroScan® range are Class IIa medical devices as defined by Directive 93/42/EEC (EC 0459) and are manufactured by Echosens™. These devices are designed for use in a medical practice in order to measure liver stiffness and ultrasound attenuation in patients with liver disease. Examinations with FibroScan® device shall be performed by an operator who has been certified by the manufacturer or its approved local representative. Operators are expressly recommended to carefully read the instructions given in the user manual and on the labelling of these products. Check cost defrayal conditions with paying bodies.
The Guided VCTE™ technology is only available on the latest FibroScan® systems and can be used only with M+ and XL+ probes.

The non-invasive gold standard solution for comprehensive management of liver health

Cloud-based solution to assist clinicians in providing comprehensive liver care.
Optimize clinical workflows with real-time secure data transmission
Your everyday FibroScan® companion – free application