
FibroScan® by echosens
The non-invasive gold standard solution for comprehensive management of liver health
Enhance FibroScan® capabilities for improved management of fatty liver patients


Improve reliability in the diagnosis and monitoring of steatosis
With SmartExam, a new computation method allows continuous measurement of CAP™ during the entire examination. In addition, when CAP™ measurement doesn’t meet the quality criteria they are automatically rejected.
SmartExam with Continuous CAP™ decreases variability by 50%.1
Continuous CAP™ can be measured on children with the S+ probe* to prevent them from undergoing biopsy.
Extend usage among severely obese patients
With SmartExam, the maximum recommended PCD (Probe to Capsula Distance) is 45 mm (vs 35 mm without SmartExam) representing an increase of 28%.
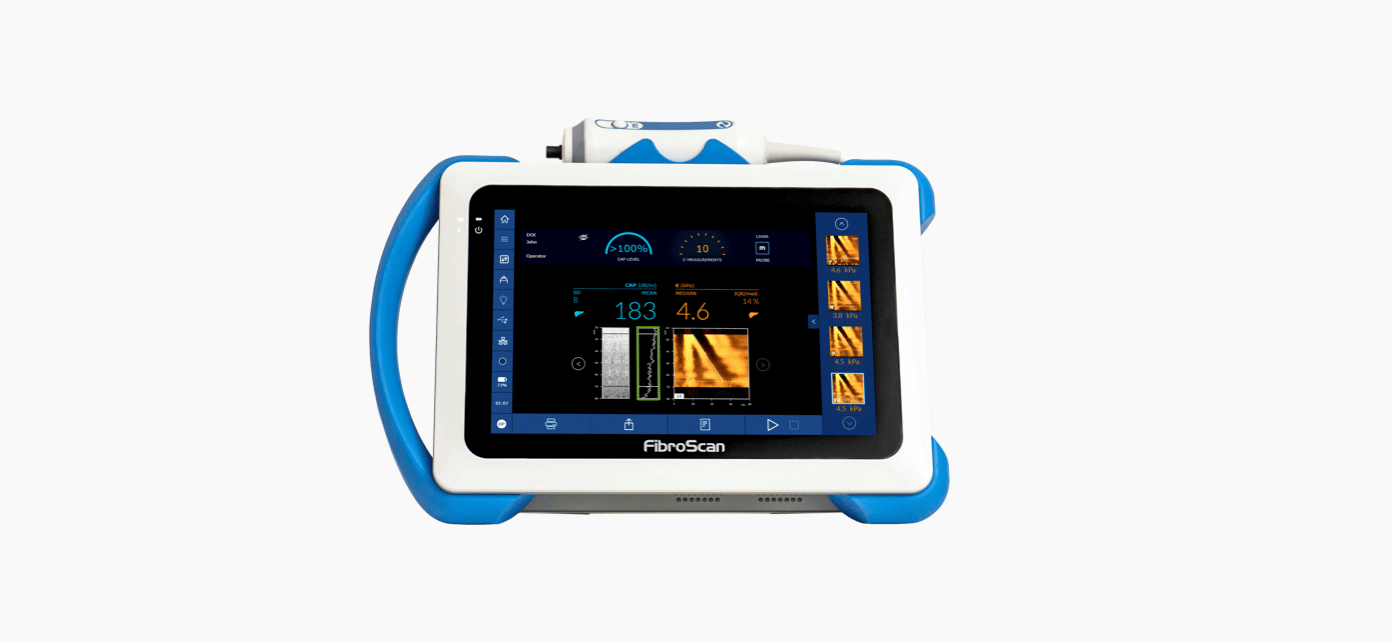
E (liver stiffness) and CAP™ gauges automatically increase when measurements are validated.
Measurement depths are automatically adapted to patient’s morphology
Operator gets notified when liver is too deep.
These task automation features were designed to further enhance the standardization of the FibroScan® examination.
SmartExam is available as an option.
Products in the FibroScan® range are Class IIa medical devices as defined by Directive 93/42/EEC (EC 0459). These devices are designed for use in a medical practice in order to measure liver stiffness and ultrasound attenuation in patients with liver disease. Examinations with FibroScan® device shall be performed by an operator who has been certified by the manufacturer or its approved local representative. Operators are expressly recommended to carefully read the instructions given in the user manual and on the labelling of these products. Check cost defrayal conditions with paying bodies.
*CAP™ is not available on S+ probe in all countries.
1.SmartExam: E. Bardou-Jacquet et al, Validation of the Continuous Controlled Attenuation Parameter (CAPc) using the MRI-PDFF as reference. EASL 2022 poster #FRI-228.

The non-invasive gold standard solution for comprehensive management of liver health

Seamless liver health assessment for all

Cloud-based solution to assist clinicians in providing comprehensive liver care.

Enhance FibroScan® liver disease assessment with biological markers