
FibroScan® by echosens
The non-invasive solution for comprehensive management of liver health
Enhance FibroScan® capabilities for improved management of fatty liver patients


Improve reliability in the diagnosis and monitoring of steatosis
With SmartExam, a new computation method allows continuous measurement of CAP™ during the entire examination. In addition, when CAP™ measurement doesn’t meet the quality criteria they are automatically rejected.
SmartExam with Continuous CAP™ decreases variability by 50%.1
Continuous CAP™ can be measured on children with FibroScan® S probe to prevent them from undergoing biopsy.
Extend usage among severely obese patients
With SmartExam, the maximum recommended PCD (Probe to Capsula Distance) is 45 mm (vs 35 mm without SmartExam) representing an increase of 28%.
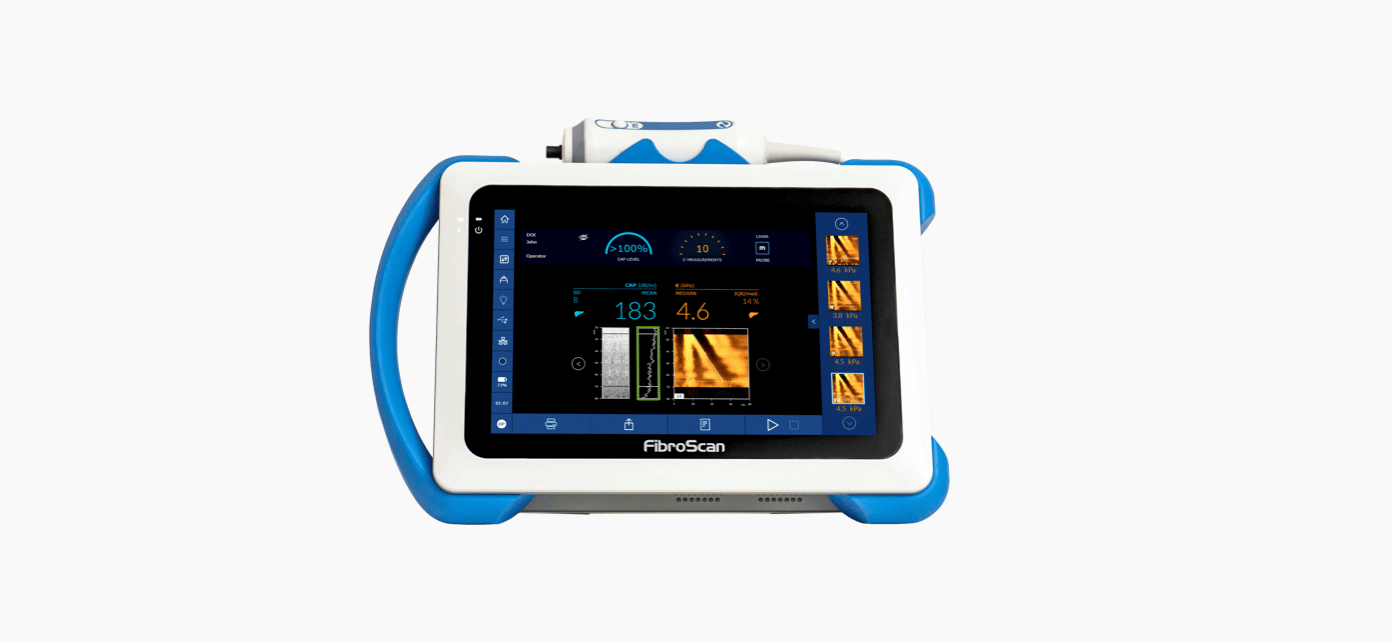
These task automation features were designed to further enhance the standardization of the FibroScan® examination.
SmartExam is available as an option.
1. E. Bardou-Jacquet et al, Validation of the Continuous Controlled Attenuation Parameter (CAPc) using the MRI-PDFF as reference. EASL 2022 poster #FRI-228
FibroScan® Indications for Use
The FibroScan® Family of Products (models: Compact 530, Mini+ 430 and Expert 630) is intended to provide shear wave speed measurements and estimates of tissue stiffness as well as ultrasound coefficient of attenuation (CAP: Controlled Attenuation Parameter) in internal structures of the body. The Shear wave speed and stiffness measurements may be used as an aid to clinical management of adult patients with liver disease.
The FibroScan® 630 is indicated for non-invasive measurement in the liver of 50 Hz shear wave speed and estimates of stiffness as well as determining a 3.5 MHz ultrasound coefficient of attenuation (CAP: Controlled Attenuation Parameter). The shear wave speed and stiffness, and CAP may be used as an aid to diagnosis and monitoring of adult patients with liver disease, as part of an overall assessment of the liver. Shear wave speed and stiffness, and CAP* may be used as an aid in the clinical management of pediatric patients with liver disease. FibroScan® 630 (Expert) is also indicated for noninvasive measurement in the spleen of 100 Hz shear wave speed and estimates of stiffness that may be used as an aid to diagnosis, monitoring and clinical management of adult patients with liver disease, as part of an overall assessment of the liver.
* CAP for pediatric patients with liver disease is only available with SmartExam capability.
FAST and Interpretation Guide are Non-Device CDS (Clinical Decision Support) that meet all parts of the 4 sections 520(o)(1)(E) criteria.

The non-invasive solution for comprehensive management of liver health

Seamless liver health assessment for all

Cloud-based solution to assist clinicians in providing comprehensive liver care.

Enhance FibroScan® liver disease assessment with biological markers.