
Guided VCTE™
Seamless liver health assessment for all
The portable non-invasive solution for efficient liver disease management
Powered by LSM by VCTE™ and CAP™ for liver fibrosis and liver steatosis assessment.


With its optional roll stand, 15” interactive touchscreen display and durable ergonomic design, FibroScan® Compact 530 is the perfect liver assessment solution for any point-of-care setting.
FibroScan® Compact 530 is easy to handle and transport (10 kg). It is a battery-powered device and is provided with transport case.
As an option, the dedicated roll stand provides a lockable drawer for probes, and a hard washable keyboard.
FibroScan® Compact 530 is adapted to all patient morphologies as it can be used with all probes S+, M+ and XL+. The two probe connectors allows to easily switch between probes during exam.
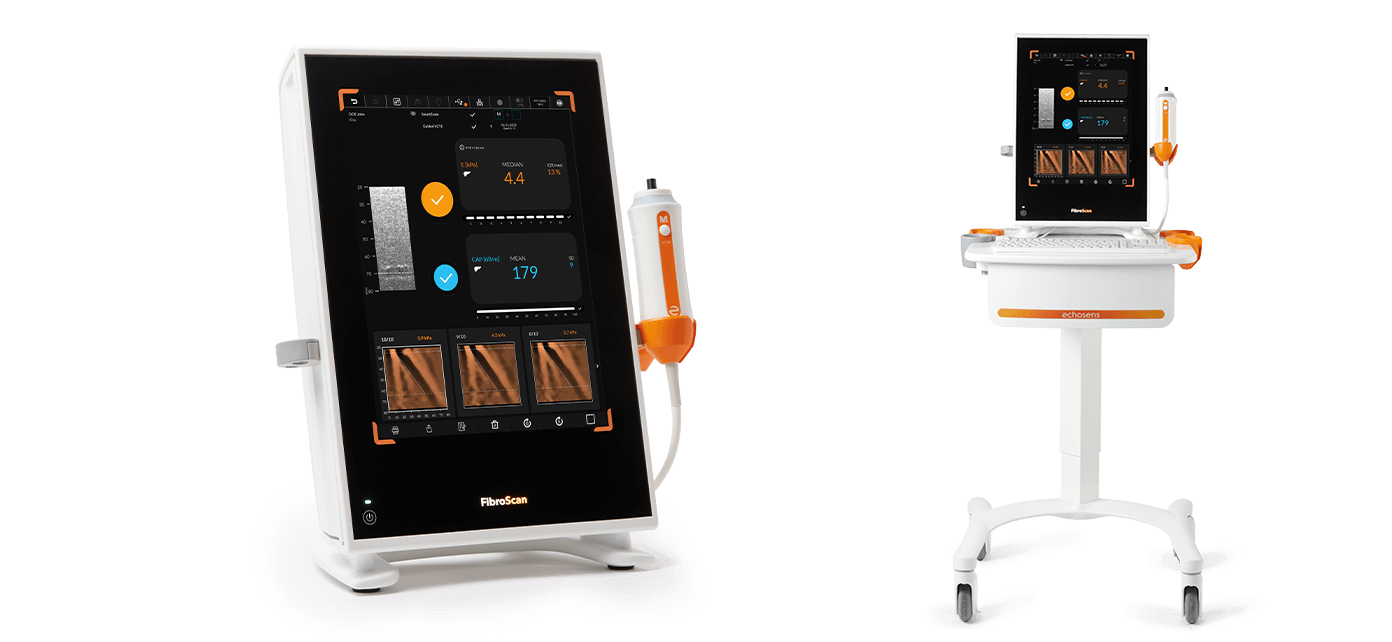
LSM by VCTE™ is unique, patented and validated for liver fibrosis assessment. It is the standard for non-invasive evaluation of liver stiffness1 with 4,778+ international peer-reviewed publications.
CAP™ is unique patented and validated for liver steatosis assessment2,3 with 1,653+ international peer-reviewed publications.
From installation, to training and local support, we provide you with the highest quality of services.
1.European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237-264. doi:10.1016/j.jhep.2015.04.006.
2.Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022-1030. doi:10.1016/j.jhep.2016.12.022.
3.Recio E, Cifuentes C, Macías J, et al. Interobserver concordance in controlled attenuation parameter measurement, a novel tool or the assessment of hepatic steatosis on the basis of transient elastography. Eur J Gastroenterol Hepatol. 2013;25(8):905-911.doi:10.1097/MEG.0b013e32835f4c3d.
FibroScan® 530 Compact is a class IIa medical devices according to Rule 10 of ANNEX VIII of Regulation EU 2017/745 (CE 0459) and is manufactured by Echosens™. FibroScan® 530 Compact is intended to provide Liver stiffness measurements at a shear wave frequency of 50 Hz and Liver ultrasound attenuation measurements (CAP: Controlled Attenuation Parameter) at 3.5 MHz. FibroScan® 530 Compact is a non-invasive device intended to aid clinical management, diagnosis, and monitoring of patients with confirmed or suspected chronic liver disease, as part of an overall assessment of the liver. FibroScan® device may aid the healthcare professionals in the assessment of liver fibrosis, steatosis, and in determining the likelihood of cirrhosis. FibroScan® device is used, in conjunction with other clinical and laboratory data, during liver assessment in patients with confirmed or suspected chronic liver disease. Examinations with FibroScan® device shall be performed by an operator who has been certified by the manufacturer or its approved local representative. Operators are expressly recommended to carefully read the instructions given in the user manual and on the labelling of these products. Check cost defrayal conditions with paying bodies.

Seamless liver health assessment for all

Optimize measurement accuracy on all patient morphologies

Cloud-based solution to assist clinicians in providing comprehensive liver care.
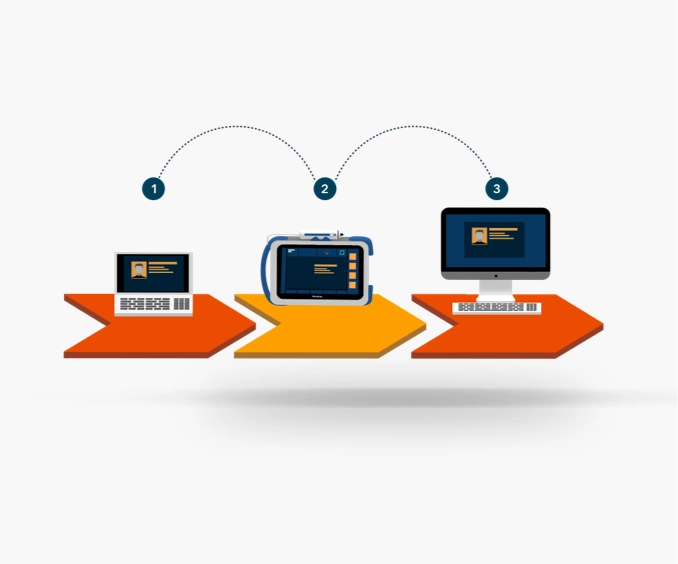
Optimize clinical workflows with real-time secure data transmission