
FibroScan® by echosens
The non-invasive solution for comprehensive management of liver health
CAP™ is the non-invasive reference parameter to diagnose and monitor liver steatosis.

There is a silent epidemic of fatty liver disease in the general population, called MASLD/MASH. The main causes of fatty liver disease are excessive alcohol consumption and/or poor diet and sedentary lifestyle.
If patients remain undiagnosed and untreated, they may go on to develop irreversible cirrhosis of the liver. If treated early with diet and lifestyle changes, the disease can be reversible.
Most patients with the presence of a metabolic syndrome.
Risk factors and predictors of MASLD
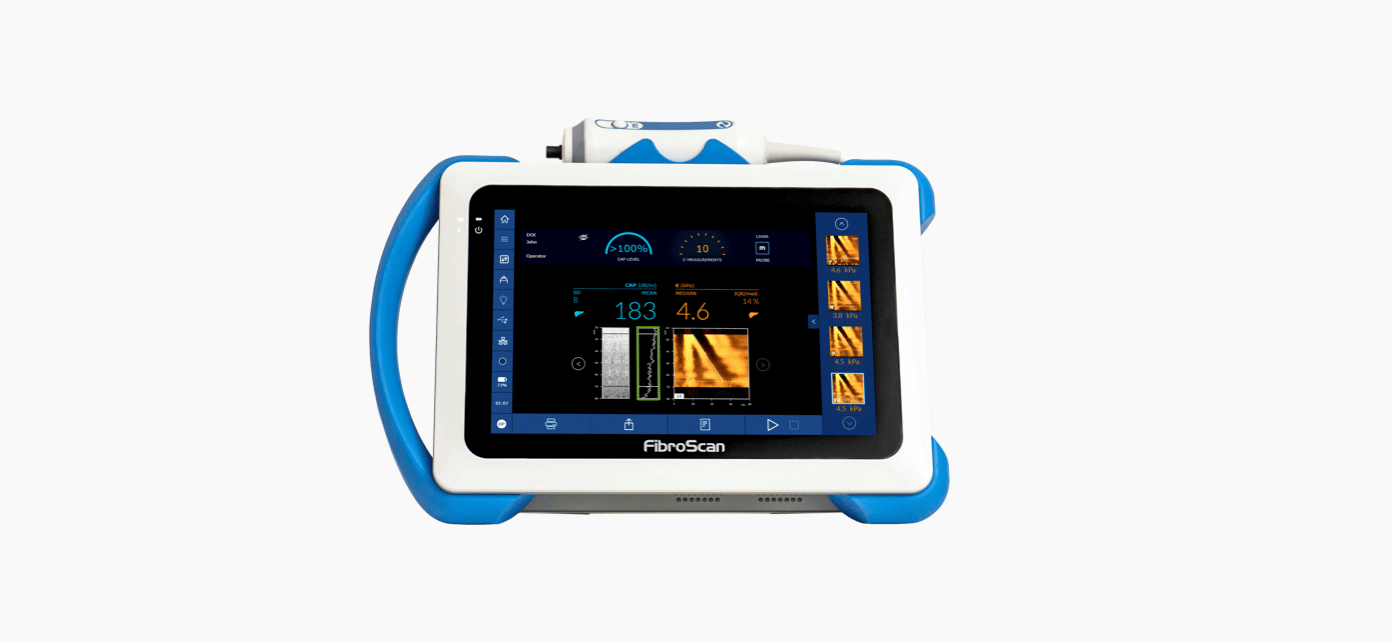
CAP™ is unique, patented and validated for liver disease management1,2. 1,130 international and peer-reviewed articles support the use of CAP™.
CAP™ is a guiding point for doctors and patients to improve monitoring of lifestyle change and therapeutic intervention.
CAP™ is a quantitative surrogate of liver steatosis expressed in decibel per meter (db/m).
Both LSM by VCTE™ and CAP™ are measured simultaneously without lengthening the examination time.
CAP™ is available on the three FibroScan® probes (S+*, M+ and XL+).
*CAP™ is not available on S probe in all countries.
1. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022-1030. doi:10.1016/j.jhep.2016.12.022.
2. Recio E, Cifuentes C, Macías J, et al. Interobserver concordance in controlled attenuation parameter measurement, a novel tool or the assessment of hepatic steatosis on the basis of transient elastography. Eur J Gastroenterol Hepatol. 2013;25(8):905-911.doi:10.1097/MEG.0b013e32835f4c3d.
Indications for use
The Shear wave speed and stiffness measurements may be used as an aid to clinical management of adult patients with liver disease.
The shear wave speed and stiffness, and CAP may be used as an aid to diagnosis and monitoring of adult patients with liver disease, as part of an overall assessment of the liver. Shear wave speed and stiffness, and CAP* may be used as an aid in the clinical management of pediatric patients with liver disease.
* CAP for pediatric patients with liver disease is only available with SmartExam capability.

The non-invasive solution for comprehensive management of liver health

Seamless liver health assessment for all

Cloud-based solution to assist clinicians in providing comprehensive liver care.

Enhance FibroScan® liver disease assessment with biological markers.