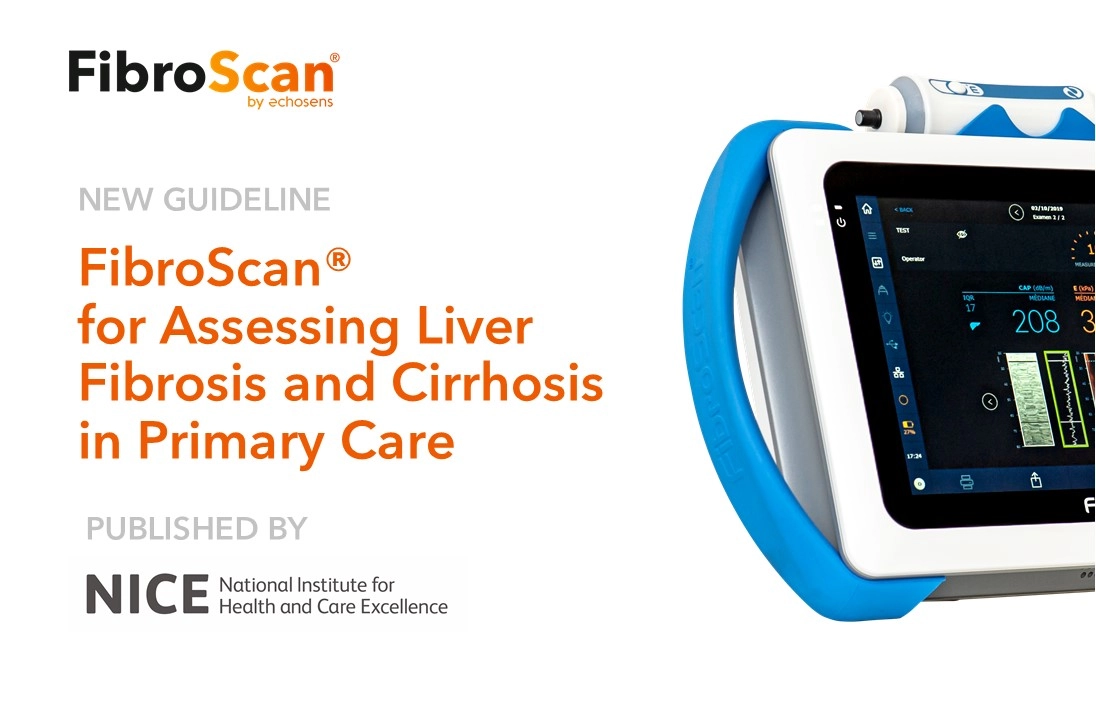
FibroScan® is a non-invasive medical device that assesses liver fibrosis and cirrhosis by measuring the degree of liver stiffness using proprietary vibration controlled transient elastography (VCTE) and a proprietary controlled attenuation parameter.
Transient elastography is the first test recommended for liver disease in adults newly referred for assessment, with liver biopsy offered based on the VCTE score, according to the National Institute for Health and Care Excellence (NICE). It is also recommended for the diagnosis of cirrhosis and either transient elastography or acoustic radiation force impulse imaging for the diagnosis of cirrhosis in people with non-alcoholic fatty liver disease (NAFLD) and advanced liver fibrosis. Liver biopsy should be considered for the diagnosis of cirrhosis in people when transient elastography is not available.
Patients at increased risk of liver cirrhosis include those who have hepatitis B and C, misuse alcohol, obesity and Type 2 diabetes.
Key Findings from the Lancet Study
Early identification and management of liver disease is an essential prerequisite to improve outcomes and avoid complications in NAFLD and alcohol-related liver disease. Primary care must play a central role in identifying patients at risk and in providing initial interventions, although mobilizing primary care at the appropriate scale remains challenging given other perceived priorities.
Researchers of the Lancet study point to a more portable, miniature FibroScan® machine for improving access for general practitioner (GP) surgeries and community centers in the United Kingdom. An unpublished pilot study initiated in early 2019 in the Mid-Hampshire clinical commissioning group—the first to make the FibroScan® available for GPs to use in primary care—has allowed better detection of the high-risk category.
Battling Alcoholic Liver Disease
The Lancet study also points to a pilot project in Edinburgh that demonstrated that FibroScan® use in an alcohol treatment setting is feasible and worthwhile. In Wales, the Gwent Liver Unit was commissioned to undertake a pilot study in which patients routinely had their liver enzyme concentrations measured and were referred for a FibroScan® if their aspartate aminotransferase:alanine aminotransferase ratio was more than 1.
From 2016–18, 18,000 people were risk-assessed and advanced fibrosis was identified in 192 individuals. This testing resulted in an 81% increase in the diagnosis of cirrhosis. This pilot has formed the backbone of a new Welsh liver blood test pathway that incorporates assertive fibrosis testing – to be launched post-COVID-19 pandemic.
Researchers determined that technologies for the pre-symptomatic diagnosis of liver disease, such as FibroScan® and serum fibrosis markers, are now ready for wide implementation in primary and community care throughout the United Kingdom as part of clear and locally owned care pathways.