
FibroScan® Probes
Choose the right probe for the right patient type
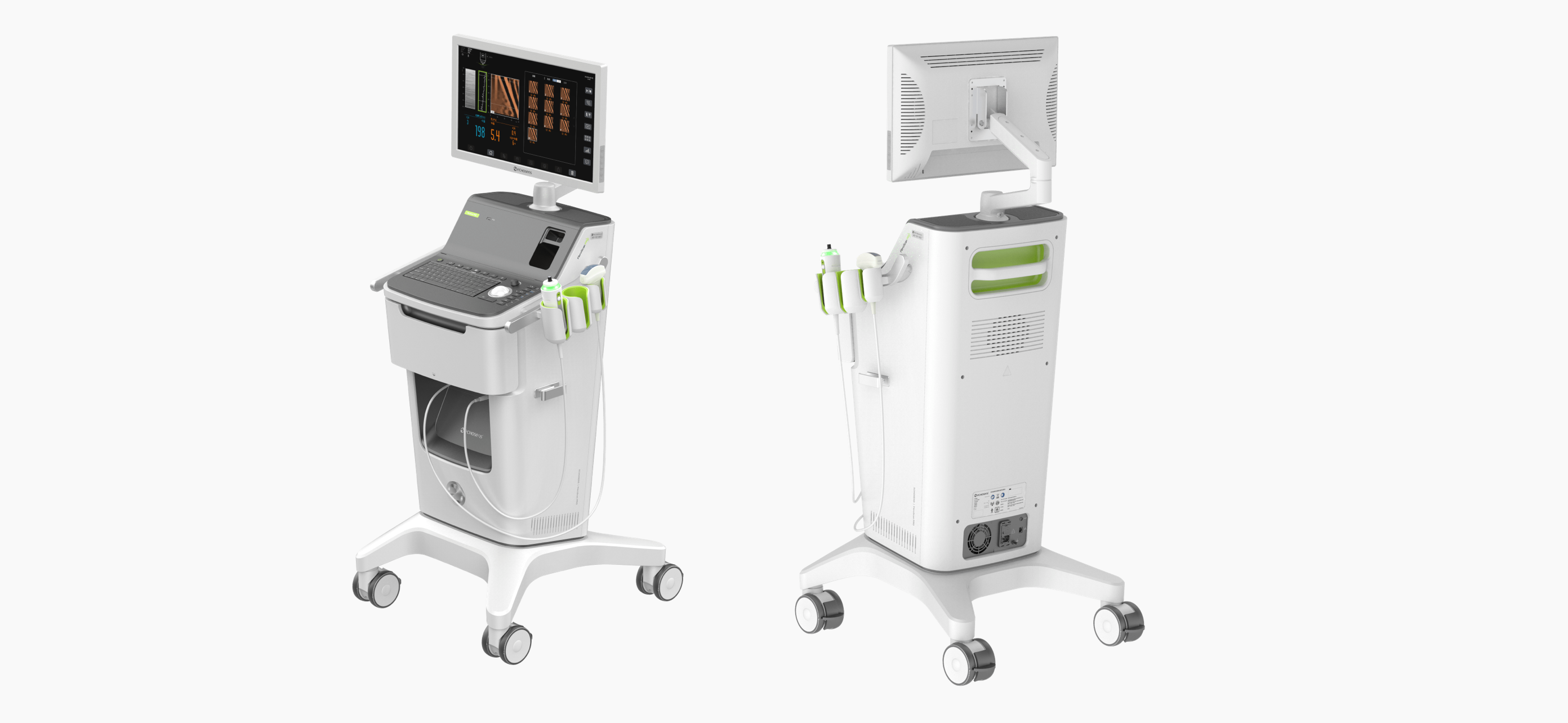
The complete non-invasive solution for advanced liver disease assessment, with embedded Ultrasound Guidance option to pre-locate the liver position.
Powered by LSM by VCTE™ and CAP™ for liver fibrosis and liver steatosis assessment.


FibroScan PRO® is designed for specialized centers in mid/large hospitals with heavy examination needs and managing complex/advanced cases.
The C-FGP probe is used to pre-locate an optimal measurement area in the liver.
LSM by VCTE™ is unique, patented and validated for liver fibrosis assessment. It is the standard for non-invasive evaluation of liver stiffness with 3,800 international peer-reviewed publications.1
CAP™ is unique patented and validated for liver steatosis assessment2,3 with 1,130 international peer-reviewed publications.
Products in the FibroScan® range are Class IIa medical devices as defined by Directive 93/42/EEC (EC 0459). These devices are designed for use in a medical practice in order to measure liver stiffness and ultrasound attenuation in patients with liver disease. Examinations with FibroScan® device shall be performed by an operator who has been certified by the manufacturer or its approved local representative. Operators are expressly recommended to carefully read the instructions given in the user manual and on the labelling of these products. Check cost defrayal conditions with paying bodies.
References are available in our bibliography.