
Baveno VII Renewing Consensus
- Update 2021 -
Personalized care in portal hypertension

Recommendations related to FibroScan®
These new Baveno VII guidelines further reinforce the use of FibroScan® for the management of patients with advanced chronic liver disease.
Key takeways
➜ LSM by VCTE™ is presented as the cornerstone NIT for both better risk stratification and improved clinical decision making for patients at risk of cACLD, CSPH and esophageal varices.
➜ LSM by VCTE™ is now also recommended for the monitoring of cACLD patients, with clear cut-offs provided to identify substantially reduced risk of decompensation and liver-related death.
➜ Like recent EASL guidelines, Baveno VII new guidelines are also introducing recommendations on spleen stiffness, with for the first-time cut-offs for SSM by VCTE™.
➜ All these cut-offs are specific to our FibroScan® technologies, providing an even stronger level of support for the use of our solutions in specialized centers.
➜ These Baveno VII guidelines will have a global impact, being endorsed by many societies across the world including EASL and AASLD.
➜ Same as Baveno VI, there is no mention of cut-offs by other shear wave elastography technologies (2D-SWE & pSWE) in these new Baveno VII guidelines, on both liver stiffness & spleen stiffness.
Clear cut-off values for LSM by VCTE™ to rule out and to rule in advanced CLD patients
• LSM values by TE <10 kPa in the absence of other known clinical/imaging signs rule out cACLD; values between 10 and 15 kPa are suggestive of cACLD;
values >15 kPa are highly suggestive of cACLD.
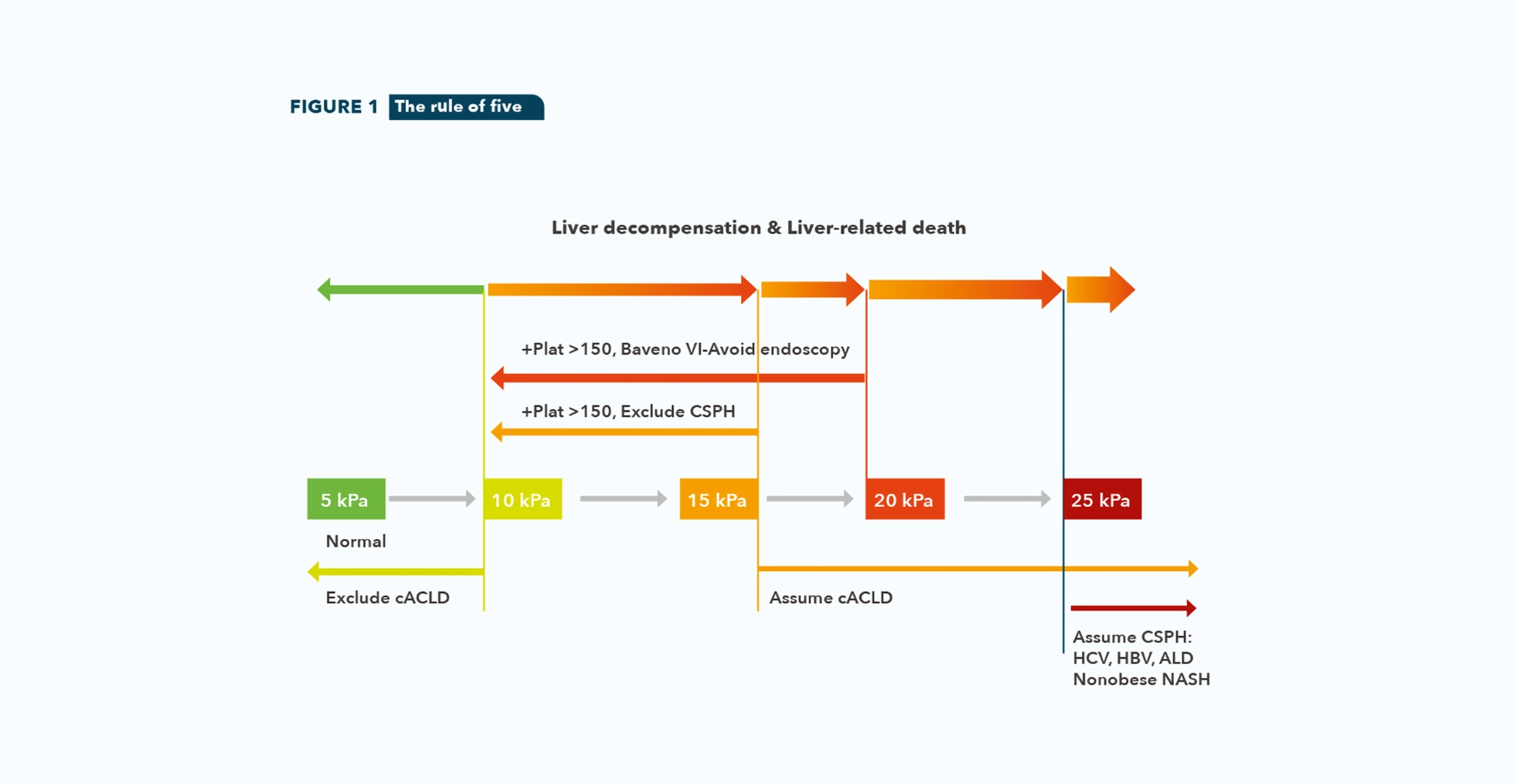
• A rule of five for LSM by TE (10-15-20-25 kPa) should be used to denote progressively higher relative risks of decompensation and liver-related death independently of the etiology of CLD. (See figure 1)
• LSM by TE ≤15 kPa plus platelet count ≥ 150×109/L rules out CSPH in cACLD patients.
• In patients with virus and/or alcohol related cACLD and non-obese (BMI <30 kg/m2) NASH cACLD, a LSM value by E ≥25 kPa is sufficient to rule in CSPH.
• Patients with compensated cirrhosis not candidates for initiating NSBB for the prevention of decompensation should undergo an endoscopy for variceal screening if LSM by TE ≥20 kPa or platelet count ≤150×109/L.
LSM by VCTE™ recommended to monitor cACLD patients
• LSM could be repeated every 12 months to monitor changes.
• A clinically significant decrease in LSM, which is associated with substantially reduced risk of decompensation and liver-related death, can be defined as a decrease in LSM of ≥20% associated with LSM<20 kPa or any decrease to a LSM<10 kPa.
• Patients avoiding screening endoscopy can be followed up by yearly repetition of TE and platelet count. If LSM increases (≥ 20 kPa) or platelet count declines (≤150×109/L), these patients should undergo screening endoscopy.
New recommendations on spleen stiffness: SSM by VCTE™
• SSM using TE can be used in cACLD due to viral hepatitis (untreated HCV; untreated and treated HBV) to rule-out and rule-in CSPH (SSM<21 kPa and SSM>50 kPa, respectively).
• SSM ≤40 kPa by TE can be used to identify subject at low probability of high-risk varices, in whom endoscopy can be avoided.
Acronyms |
Endorsement |
|
cACLD: Compensated Advanced Chronic Liver Disease, this term had been proposed to reflect the continuum of severe fibrosis and cirrhosis in patients with ongoing CLD. CLD: Chronic Liver Disease CSPH: Clinically Significant Portal Hypertension LSM: Liver Stiffness Measurement NASH: Non-alcoholic Steato-hepatitis NIT: Non-invasive Test NSBB: Nonselective Beta Blocker PH: Portal Hypertension SSM: Spleen Stiffness Measurement TE: Transient Elastography VCTE: Vibration Controlled Transient Elastography |
The Baveno VII Consensus workshop was endorsed and supported with unrestricted grants by the following: a) International Scientific Societies: EASL (European Association for the Study of the Liver) b) national scientific societies: AASLD (American Association for the Study of Liver Disease); AEEH (Spanish Association for the Study of the Liver), AFEF (French Association for the Study of the Liver), AIGO (Italian Association of Hospital Gastroenterologists and Endoscopists); AISF (Italian Association for the Study of the Liver); CIBERehd (Spanish network of biomedical investigation in liver and digestive diseases), ÖGGH (Austrian Society for Gastroenterology and Hepatology), SASL (Swiss Association for the Study of the Liver), SIGE (Italian Society of Gastroenterology). |


