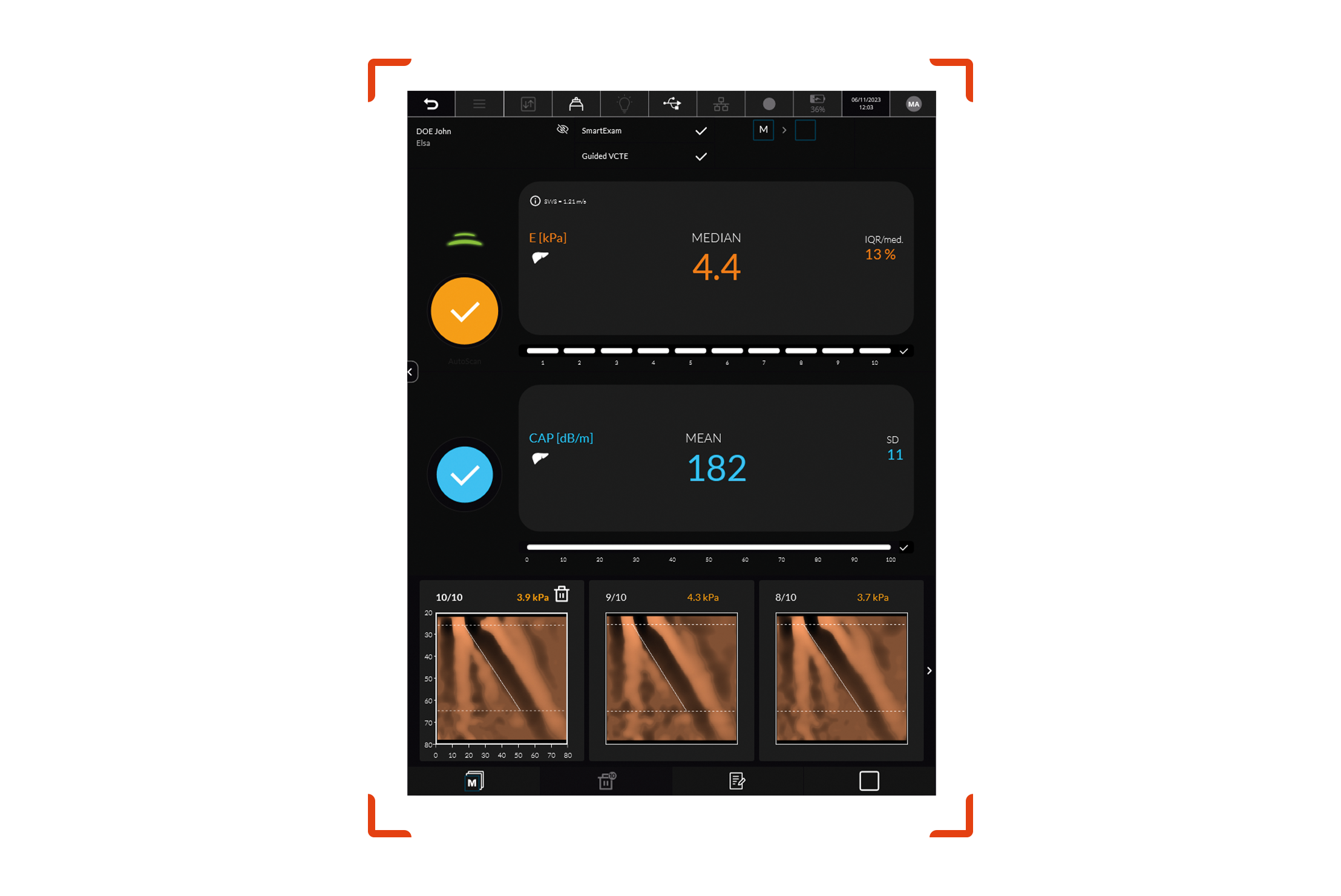
This milestone opens a path for FibroScan® device with its VCTE™ probe and elastography system, using vibration-controlled transient elastography technology to ultimately replace liver biopsy for patient enrollment and treatment response assessment in MASH drug development. Echosens’ achievement will make participation in clinical trials less burdensome and more accessible for people living with advanced steatotic liver disease.
Echosens is particularly proud to have accomplished this major advancement, which paves the way for significantly facilitating research for the development of remedies designed to combat the scourge of liver diseases and thus ultimately contributing to improving patient care. This acceptance recognizes Echosens’ more than 20-year investment to validate the standardized data generated by its FibroScan® device and certify this equipment and its proprietary technology with clinical research teams. This investment enabled FibroScan® and its VCTE™ probe and elastography system to be cited in more than 5,600 peer-reviewed publications, including recent pivotal publications (Gawrieh et al., Journal of Hepatology, 2024, Lin et al., JAMA, 2024, and Mózes et al., Lancet Gastroenterol Hepatol, 2023), and in more than 200 international clinical practice guidelines.
FDA’s acceptance of Echosens’ Letter of Intent applies only to Liver Stiffness Measurement (LSM) measured by vibration-controlled transient elastography, therefore exclusively based on data obtained with FibroScan® devices equipped with its VCTE™ probe and elastography system. Other equipment suppliers using different methods to measure LSM for this indication cannot claim that FDA’s recent Acceptance Determination Letter applies to their equipment. They are therefore invited to submit their own Letter of Intent for DDT to the FDA based on data obtained with their equipment and technologies.
Furthermore, Echosens considers it unlawful and wrongful to associate this FDA Acceptance Determination Letter with any equipment other than Echosens’ FibroScan® device equipped with its VCTE™ probe and elastography system. In particular, the biomarkers validated by the FDA should not be confused with those obtained using other elastography techniques, including other transient elastography (TE) techniques.
FibroScan® equipment is protected by patents worldwide. Echosens is the exclusive owner of all right, title, and interest in and to all trademarks included in this statement, including VCTE, Echosens, and FibroScan. The trademarks Echosens and FibroScan are federally registered trademarks of Echosens.