
AACE Clinical Practice Guidelines
Co-Sponsored by AASLD 2022
Diagnosis and Management of Non-Alcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings

Recommendations related to FibroScan®
These new guidelines are a breakthrough in early diagnosis and management of patients with NAFLD targeting primary care and endocrinology clinical settings.
The new guidelines include 34 evidence-based clinical practice recommendations for the screening, diagnosis, and management of adults and children with NAFLD and/or NASH.
These guidelines reinforce the use of FibroScan® solutions for the management of patients with NAFLD throughout the patient care continuum.
Key Takeaways
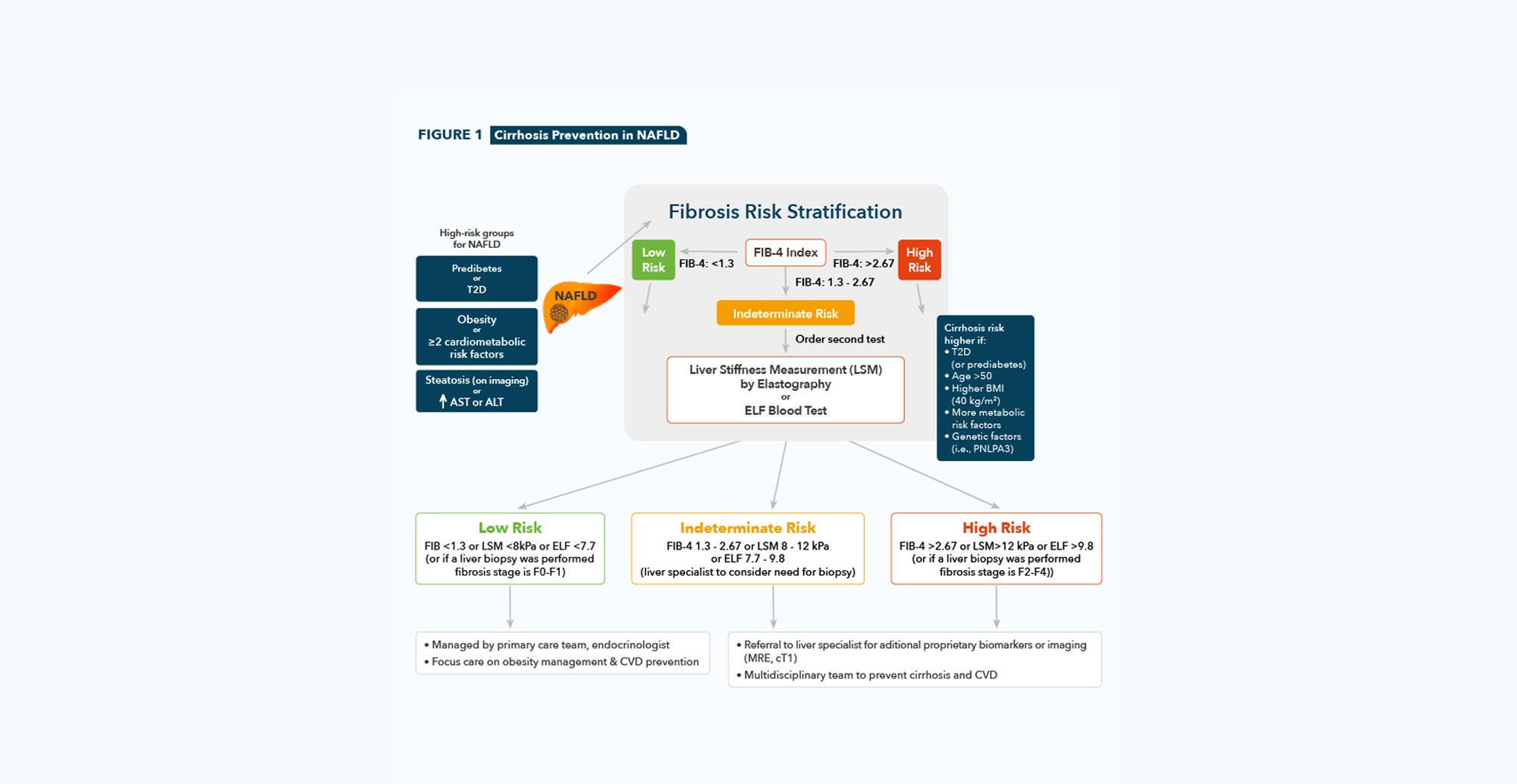
➜ LSM by VCTE™ should be used for further workup in individuals that are in the “high-risk” group who have an indeterminate or high FIB-4 score.
➜ LSM by VCTE™ is preferred as it can quantify liver fibrosis (LSM by VCTE™) and liver fat (CAP™) for risk stratification in one test.
➜ LSM by VCTE™ is best validated to identify advanced disease and predict liver-related outcomes.
Diagnosis of NAFLD in Adults
Clinicians should consider screening individuals with any of the following risk factors: obesity, metabolic syndrome, prediabetes or T2D, hepatic steatosis seen on any imaging study, and/or persistently elevated plasma aminotransferase levels (over 6 months).
Grade B; Intermediate/High Strength of Evidence; BEL 2
•
Clinicians should use liver fibrosis predictioncalculations to assess the risk of NAFLD
with liver fibrosis. The preferred noninvasive initial test is the FIB-4.
Grade B; Intermediate Strength of Evidence; BEL 2
Evidence Based: Hepatic steatosis can be diagnosed on imaging, including liver US, CAP™, computed tomography, or 1 H-MRS and magnetic resonance imaging-proton density fat fraction (MRI-PDFF).
•
Clinicians should consider individuals belonging to the “high-risk” group who have
an indeterminate or high FIB-4 score for further workup with an LSM by VCTE™ (transient elastography) or ELF test.
Grade B; Intermediate Strength of Evidence; BEL 2
Evidence Based: Hepatic steatosis may be assessed by means of simple non-invasive liver
steatosis scores (fatty liver index, US fatty liver index, and hepatic steatosis index), although these diagnostic modalities have inherent limitations.
A liver US is not recommended for routine clinical diagnosis. Instead, LSM by VCTE™ is preferred over liver US, where available, as it can quantify liver fat (CAP™) and fibrosis (LSM by VCTE™) for risk stratification in one test.
•
To stage the risk of fibrosis in individuals with NAFLD, clinicians should prefer the use of LSM by VCTE™ as best validated to identify advanced disease and predict liver-related outcomes.
Grade B; Intermediate Strength of Evidence; BEL 2
•
Individuals with T2D, clinicians should consider screening for clinically significant fibrosis (stages F2-F4) using the FIB-4, even if they have normal liver enzyme levels.
Grade B; High/Intermediate Strength of Evidence; BEL 2
•
Individuals with T1D, clinicians may consider screening for NAFLD with clinically significant fibrosis (stages F2-F4) using the FIB-4, only if there are risk factors such as obesity, metabolic syndrome, elevated plasma aminotransferase levels (>30 U/L), and/or hepatic steatosis on imaging.
Grade C; Intermediate/Weak Strength of Evidence; BEL 2; downgraded based on the heterogeneity of studies and moderate to high probability of bias
•
Clinicians should further risk stratify individuals with T2D, or T1D with cardiometabolic risk factors and/or elevated plasma aminotransferase levels (>30 U/L) using the FIB-4, LSM by VCTE™, and/or ELF test.
Grade B; High/Intermediate Strength of Evidence; BEL 2
•
Individuals with persistently elevated ALT or AST levels and/or with hepatic steatosis on imaging and indeterminate risk (FIB-4, 1.3-2.67; LSM by VCTE™, 8-12 kPa; or ELF test, 7.7-9.8) or high risk (FIB-4, >2.67; LSM by VCTE™, >12 kPa; or ELF test, >9.8) based on blood tests and/or imaging should be referred to a gastroenterologist or hepatologist for further assessment, which may include a liver biopsy.
Grade B; Intermediate Strength of Evidence; BEL 2
Evidence Based: The frequency of testing for individuals considered at low risk based on the
FIB-4 (<1.3) or LSM by VCTE™ (<8.0 kPa) is not as well established as it is for the “high-risk”
groups; however, it is prudent to consider repeat testing every 2 years for those at low risk,
as 1 study[1] showed that only a minority will progress to a higher fibrosis stage within
that period of time (<20%).
Management of NAFLD in Adults
• Clinicians must consider treating diabetes with pioglitazone and/or GLP-1 RAs when there is an elevated probability of having NASH based on elevated plasma aminotransferase levels and noninvasive tests.
Grade A; High Strength of Evidence; BEL 1
Evidence Based: in high-risk populations (i.e.,those with obesity and T2D), pharmacologic
therapy to treat obesity or diabetes may also be considered in the presence of elevated
plasma aminotransferase levels and/or FIB-4 scores of >1.3 and confirmatory imaging (i.e.,
LSM by VCTE™ and MRE) or proprietary fibrosis biomarkers, such as the ELF test, when suggestive of clinically significant liver fibrosis, if imaging not available.
Diagnosis and Management of Children with NAFLD
Children of any age and adolescents with obesity or T2D, but not T1D, should be screened for NAFLD using serum ALT.
Grade B; Intermediate/High Strength of Evidence; BEL 2
•
Clinicians should screen adolescent females with polycystic ovary syndrome for NAFLD using serum ALT.
Grade B; Intermediate/High Strength of Evidence; BEL 2
•
Pediatric NAFLD can be diagnosed with imaging (ultrasound or magnetic resonance imaging-protondensity fat fraction) or liver biopsy, in combination with exclusion of non-NAFLD causes of hepatic steatosis such as Wilson syndrome, mitochondrial disease, and medications.
Grade B; Intermediate Strength of Evidence; BEL 2
•
Liver fibrosis prediction calculations and proprietary biomarkers currently available for the diagnosis of advanced fibrosis in adults should not be used in children as they are either inaccurate or require further validation.
Grade B; Intermediate Strength of Evidence; BEL 2
Evidence Based: Other imaging approaches, such as LSM by VCTE™, are becoming more widely available; however, pediatric-specific norms have not yet been developed for NAFLD.
Grading System |
Acronyms |
| • Recommendation Grade – Grade A (strong) – Grade B (intermediate) – Grade C (weak) – Grade D (no conclusive evidence and/or expert opinion) • Strength of Evidence Grade • Best Evidence Level (BEL) – BEL 1 (strong evidence) – BEL 2 (intermediate evidence) – BEL 3 (weak evidence) – BEL 4 (no evidence) |
• AACE: American Association of Clinical Endocrinology • AASLD: American Association for the Study of Liver Diseases • CVD: Cardiovascular Disease • LSM: Liver Stiffness Measurement • VCTE: Vibration Controlled Transient Elastography • CAP: Controlled Attenuation Parameter • NAFLD: Nonalcoholic Fatty Liver Disease • T2D: Type 2 Diabetes • T1D: Type 1 Diabetes • Liver US: Liver Ultrasound • H-MRS: Proton Magnetic Resonance Spectroscopy • MRI PDFF: Magnetic Resonance Imaging – Proton Density Fat Fraction |


