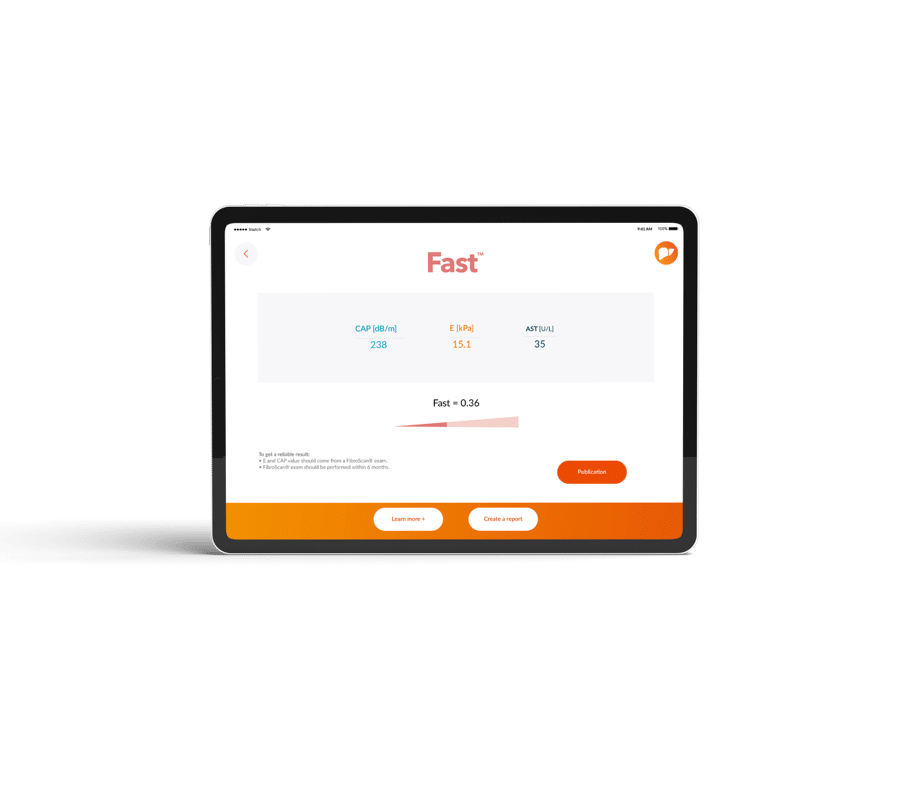
FAST™ is a cost-effective tool to help identify individuals at risk for fibrotic NASH, an asymptomatic progressive liver disease that leads to increased liver-related mortality and morbidity.
The FAST™ score is a simple combination of two physical biomarkers: liver stiffness by Vibration Controlled Transient Elastography (VCTE) and Controlled Attenuation Parameter (CAP) together with aspartate aminotransferase (AST), a circulating biomarker.
Dr. Phil Newsome, University of Birmingham Professor of Experimental Hepatology, University Hospitals Birmingham NHS Foundation Trust Consultant Hepatologist, Director of the Centre for Liver and Gastrointestinal Research, Director of the Midlands and Wales Advanced Therapy Treatment Centre, and Deputy Director of the NIHR Birmingham Biomedical Research Centre, says:
“The FAST™ score was derived from a prospective, multi-center study with 350 patients undergoing a liver biopsy and then validated in seven external cohorts with 1,026 patients. FAST score provides an efficient way to non-invasively identify at-risk patients with progressive NASH that merit consideration for further treatment. This is very timely and significant because the efficient identification of active fibrotic NASH has long been a challenge for drug companies, and many are now developing drugs targeting this disease.”
Professor Newsome points to the multiple benefits of FAST™, explaining that FAST™ is inexpensive, reduces unnecessary invasive testing and represents a quick, point-of-care solution that mitigates the need to send out for complex blood tests.
“FAST™is simple to determine and interpret – just requiring three numbers entered into a calculator,” he adds, further explaining that FAST™ score provides a probability of active fibrotic-NASH.
It is estimated that 25% of the worldwide population is affected by non-alcoholic fatty liver disease (NAFLD), of which 3% to 5% have NASH. In the US and EU, it is estimated that 12.2MM people may be living fibrotic-NASH, many of which are undiagnosed.
Dr. Stephen A. Harrison, M.D., FACP, FAASLD, COL (ret.), USA, MC, visiting professor of Hepatology, Radcliffe Department of Medicine, University of Oxford, and medical director, Pinnacle Clinical Research, says, “The simplicity of the FAST™ score makes it easy to access, inexpensive and simple to interpret. This enables front-line health care professionals to calculate the score with an online calculator, like the one found on the available myFibroScan app, making this an economical and effective way to non-invasively determine if a patient is at-risk for progressive NASH and requires further treatment.”
As Echosens continues to expand its capabilities of FibroScan, Dominique Legros, Group CEO, Echosens, states, “We envision that FAST™ score will be the first of many scores that combines the FibroScan physical biomarkers with circulating biomarkers. We look forward to making further developments for monitoring liver disease based on the consistency of FibroScan results.”