Superior to Liver Biopsy
Liver biopsy is considered the gold standard for staging the severity of fibrosis and presence of cirrhosis, but carries drawbacks, such as inter and intra-observer variability, sampling errors, unequal distribution of fibrosis in the liver, risk of complications and even death. The gold standard for assessing presence of PH is hepatic venous pressure gradient (HVPG), a highly invasive procedure performed at a limited number of centers. For these reasons, non-invasive methods have been developed to assess levels of liver fibrosis and the presence of PH. In fact, liver stiffness measured by transient elastography in combination with platelet count can rule out the presence of high-risk esophageal varices commonly associated with PH.
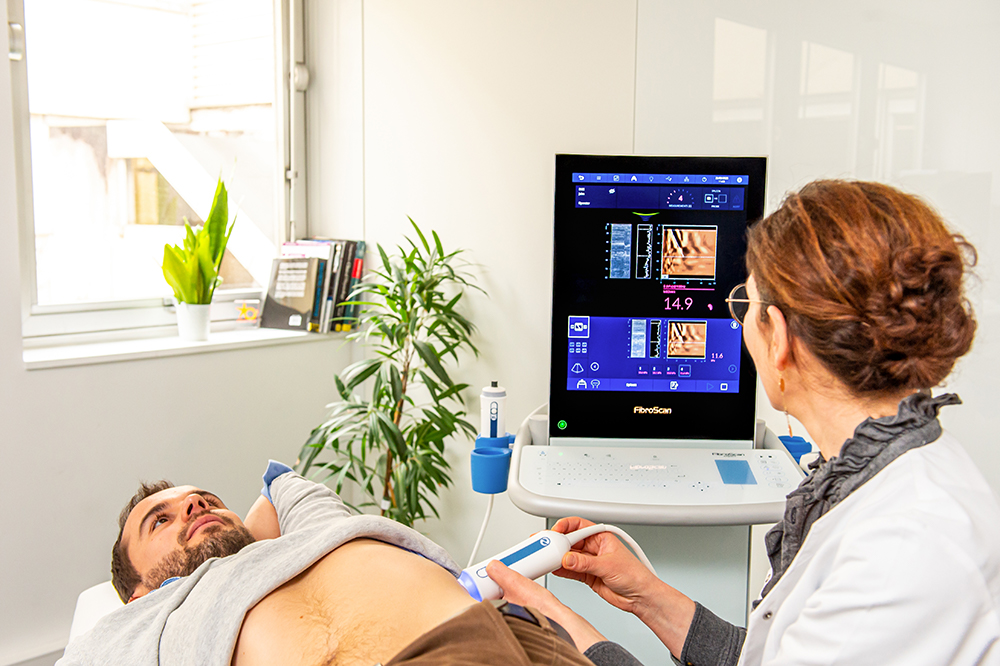
Responding the need for non-invasive diagnostics, Echosens has introduced the FibroScan® 630 Expert, a complete non-invasive solution for assessing and monitoring more advanced forms of chronic liver disease. With the addition of spleen stiffness measurement, this innovative tool is designed to expand clinical capabilities in liver health assessment to include PH.
Based on the scientific data already available, measurement of spleen stiffness can be used for non-invasive assessment and monitoring of PH and to detect esophageal varices in patients with hepatitis C virus-induced cirrhosis.. Clinicians can utilize FibroScan® 630 Expert to assess the risk of the cirrhotic patient and adapt care.
Addressing Cirrhosis in the United States
Less than 1% of the United States population have cirrhosis of liver. In the western world, the most common etiology of portal hypertension is cirrhosis due to alcoholic liver disease, NASH and hepatitis C infection.
According to a recent estimate 15 million people in the United States have alcohol abuse disorder, nearly 88,000 people die annually due to alcohol, 10%-15% of people with alcoholism develop cirrhosis, and another 3 million people have chronic hepatitis C infection, with 25%-28% of these patients developing cirrhosis.
Key Upgrades
The FibroScan® 630 Expert offers an intuitive user interface, high-speed processing, integrated barcode reader, ultrasound localization probe-time-saving technology for locating the spleen and liver in potentially complex patients. The technology offers redesigned ergonomics, touchscreen and a washable touch keyboard.
FibroScan® 630 Expert is powered by: LSM by VCTE™, patented and validated for liver disease management and supported by 2,500 peer-reviewed publications; CAP™, patented and validated for liver disease management and supported by international and peer-reviewed articles; and SSM by VCTE™, patented and validated for spleen and liver stiffness measurements as a new marker for non-invasive evaluation of spleen stiffness, which is supported by 50 peer-reviewed publications.