FibroScan®-Facilitated Treatment Options

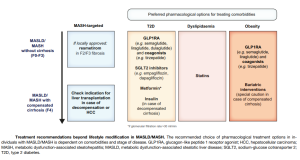
Guideline-Endorsed Treatment Options
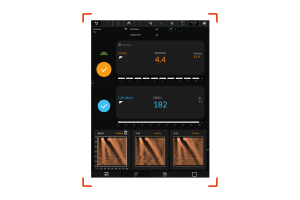
FibroScan® Measures Two Important Components of Liver Health
Liver Stiffness by VCTE™ a correlate to fibrosis and CAP™ a correlate to steatosis. These measurements are important both together as well as looked at separately along the patient’s journey.
LSM by VCTE™ may be used as an aid in the assessment of liver fibrosis.
CAP™ may be used as an aid in the assessment of hepatic steatosis.
FibroScan® is indicated as a non-invasive aid for the clinical management, diagnosis, and monitoring of adult and pediatric patients with confirmed or suspected liver disease.
VCTE™ and CAP™ both play an important role in identifying/qualifying a patient for treatment.
CAP™ is particularly of benefit to show response to REZDIFFRA™ as it will indicate a reduction in CAP™ value if there is improvement from the drug.
Thanks to our revolutionary Guided VCTE™ technology, the examination is simpler and quicker.
FibroScan® – The Only Non-Invasive Point-of-Care Solution Referenced in the FDA Prescribing Information Label for Rezdiffra™
Following REZDIFFRA™ FDA approval on March 14th, FibroScan® is the only point-of-care non-invasive test referenced in REZDIFFRA FDA prescribing information label.2
Revolutionizing Liver Disease Treatment: A Conversation with Dr. Naim Alkhouri about the First-ever Therapeutic for MASH
We recently sat down with Dr. Naim Alkhouri, Chief Medical Officer, Chief of Transplant Hepatology, and Director of the Fatty Liver Program at Arizona Liver Health to discuss a historic turning point in the fight against liver disease—the FDA’s approval of Rezdiffra™.
Dr. Alkhouri is believed to be the first physician to get a patient with fibrotic MASH approved for the drug in the U.S. The patient was identified using FibroScan® technology—the only non-invasive point-of-care solution referenced directly in the FDA prescribing information label. Thanks to its patented LSM by VCTE™ for liver fibrosis assessment and CAP™ for hepatic steatosis assessment, FibroScan® is FDA-approved for both diagnosis and ongoing monitoring of patients with suspected or confirmed liver disease.
To discuss what the new therapeutic option means for patients and the future of liver health, we asked Dr. Alkhouri to share his thoughts on the dawn of a new era in the fight against MASH and liver disease.
The Essential Role of FibroScan® in the “MAESTRO-NASH” Phase 3 trial
In “MAESTRO-NASH” Phase 3 trial supporting REZDIFFRA™ FDA approval, the proprietary biomarkers of FibroScan® (LSM by VCTE™ and CAP™) played a key role in both patient prescreening criteria and measurement of therapeutic response after 52 weeks of treatment.1
Managing MASH Patients
FibroScan® Plays an Essential Role in the Diagnosis and Management of Patients with MASLD/MASH.
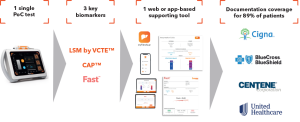
Coverage & Authorization Overview
Our FibroScan® ecosystem is providing all required documentation for providers to smoothly submit prior authorization.
References
1. Harrison, S. A., Bedossa, P., Guy, C. D. et al. (2024), ‘A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis’, N Engl J Med, 390/6: 497–509
2. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/217785s000lbl.pdf
3. same as 2 – Table 7: Baseline Characteristics in Adults Patients with Noncirrhotic NASH with Stage 2 to Stage 3 Fibrosis in Trial 1
4. same as 2 – Section 2.2 Pharmacodynamics
5. K223902.pdf (fda.gov)