A New Era in MASH Treatment: How FibroScan® Supports Patient Care
With two FDA-approved therapies now available for MASH with moderate to advanced fibrosis, FibroScan® helps connect patients to treatment by enabling early diagnosis, risk stratification, and ongoing monitoring.

|
For the first time, adults with noncirrhotic MASH and moderate to advanced fibrosis (F2–F3) have access to two FDA-approved medications. Rezdiffra™ (resmetirom) was the first drug approved for this indication, in March 2024, followed by Wegovy® (semaglutide), in August 2025, a GLP-1 therapy also indicated for weight management and cardiovascular risk reduction. These approvals mark a major turning point in liver health, creating new urgency around early identification and accurate assessment—areas where FibroScan® delivers unique value. |
 |
FDA-Approved Therapies for MASH
Rezdiffra™ is indicated in conjunction with diet and exercise for the treatment of adults with noncirrhotic MASH with moderate to advanced liver fibrosis (consistent with stages F2 to F3 fibrosis).
- This indication is approved under accelerated approval based on improvement of MASH and fibrosis. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
- Limitation of use: Avoid use in patients with decompensated cirrhosis.
Wegovy® (semaglutide) injection 2.4 mg is indicated, in combination with a reduced calorie diet and increased physical activity, for:
- Treatment of adults with noncirrhotic MASH with moderate to advanced fibrosis (F2–F3). The indication for MASH is approved under accelerated approval based on the improvement of MASH and fibrosis. Continued approval for this indication may be contingent upon the verification and description of clinical benefit in a confirmatory trial.
- Risk reduction of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established cardiovascular disease and either obesity or overweight.
- Reduction of excess body weight and maintenance of weight reduction long term in adults and pediatric patients aged 12 years and older with obesity and adults with overweight in the presence of at least one weight-related comorbidity.
- Limitations of use: Wegovy® contains semaglutide. Coadministration with other semaglutide-containing products or with any GLP-1 receptor agonist is not recommended.
Please see full prescribing information for Rezdiffra™ and Wegovy®.
The Role of FibroScan®
 |
|
FibroScan® is widely adopted in clinical practice and has been instrumental in the pivotal trials that led to the recent FDA approvals for MASH therapies.
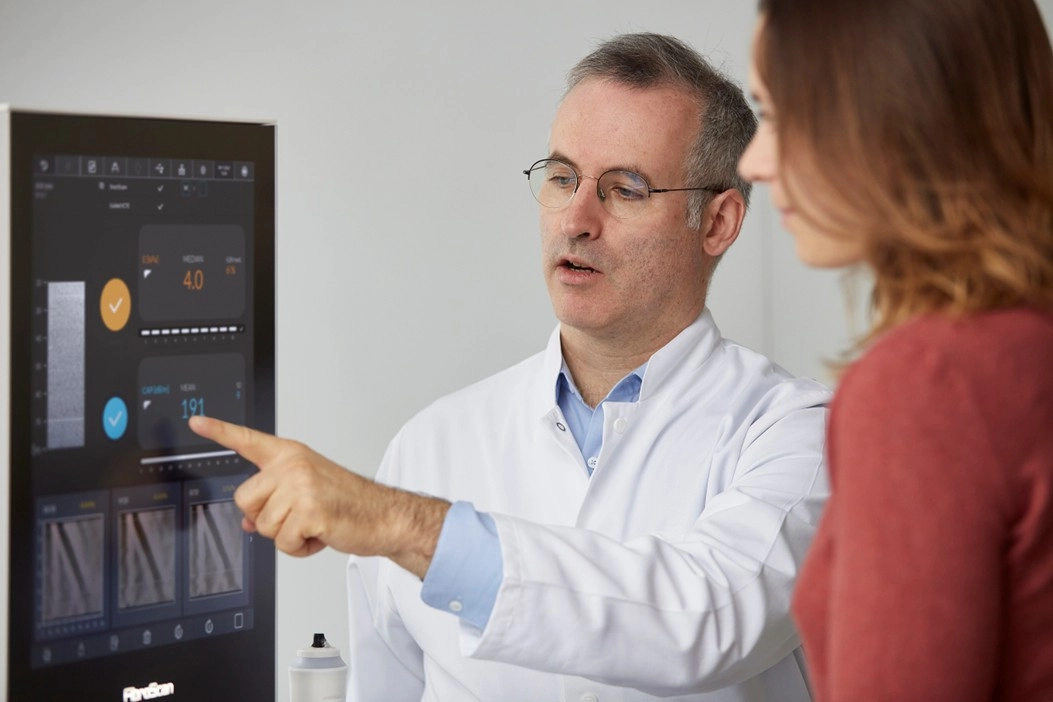
- In Novo Nordisk’s ongoing ESSENCE trial, FibroScan® is a key non-invasive tool (NIT). It was used to screen patients for enrollment and to measure secondary endpoints related to NITs. At 72 weeks, patients showed improvements in both CAP score (a measure of steatosis) and LSM by VCTE™ (an assessment of fibrosis) compared to placebo.
- In Madrigal’s MAESTRO-NASH Phase 3 trial, the proprietary biomarkers of FibroScan® (LSM by VCTE™ and CAP) played a key role. These measurements were crucial for patient prescreening and provided accurate, non-invasive assessment of therapeutic response after 52 weeks.
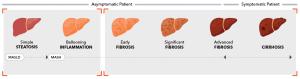
The Stealth Threat of MASH
MASH affects approximately 1 in 20 adults1, yet 9 out of 10 people2 living with it remain undiagnosed.
Without intervention, MASH can progress to cirrhosis, liver failure, and higher risk of cardiovascular events and type 2 diabetes.
Expanding access to scalable, validated, and cost-effective NITs like FibroScan® is critical to closing the diagnosis gap and connecting patients with timely care.
Bring FibroScan® into your practice
Discover how FibroScan® can support diagnosis, streamline documentation, and help your team monitor patient response to new MASH therapies.