World Health Organization
- Guidelines 2024 -
Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B (CHB) infection

Recommendations related to FibroScan®
Since the 2015 guidelines were published, several significant developments have occurred including new study data on diagnostic performance of non-invasive tests for staging liver disease and cut-off thresholds for diagnosing significant fibrosis or cirrhosis.
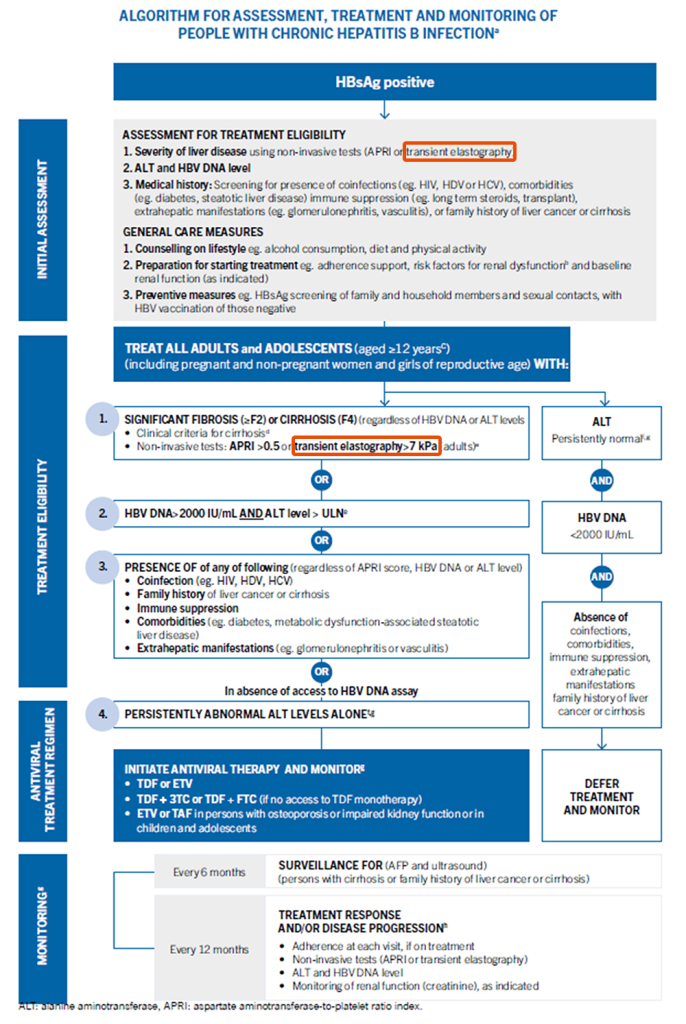
The objective of the 2024 guidelines is to provide updated evidence-informed recommendations on key priority topics. These include expanded and simplified treatment criteria for adults but now also for adolescents.
On the perspective of expanded treatment eligibility, new recommendations using Transient Elastography (TE) by FibroScan® are added to “use of non-invasive tests for staging of liver disease” at Chapter 4 & “who to treat among people with chronic hepatitis B” at Chapter 5.
Focus on Chapter 4 – Use of non-invasive tests for staging of liver disease
Recommendations
- Evidence of significant fibrosis (≥F2) should be based on TE (FibroScan®) value of >7.0 kPa
- Evidence of cirrhosis (F4) should be based on clinical criteria with TE (FibroScan®) value of >12.5 kPa.
Remarks
These cut-offs apply to FibroScan®– other elastography techniques do not necessarily have the same cut-offs.
Mentions
Since 2017, the use of TE (FibroScan®) during pregnancy is no longer contraindicated (US FDA 2023 clearance).
TE (FibroScan®) is non-invasive, takes less than 10 minutes to perform and can be undertaken in outpatient or community settings.
Medical, nursing and other healthcare personnel can be easily trained to use FibroScan®.
Focus on Chapter 5 – Who to treat among people with chronic hepatitis B
Treatment is recommended for all adults and adolescents (aged ≥12 years) with CHB
(including pregnant women and girls and women of reproductive age).
Transient elastography by FibroScan® is one of the options for treatment eligibility.
Recommendation
- Treatment is recommended with evidence of significant fibrosis (≥F2) based on TE (FibroScan®) value >7 kPa
- or evidence of cirrhosis (F4) based on clinical criteria with TE (FibroScan®) value of >12.5 kPa, regardless of HBV DNA or ALT levels
TE (FibroScan®) is recommended at initial assessment and treatment eligibility of people with chronic hepatitis B infection