
FibroScan® Probes
Optimize measurement accuracy on all patient morphologies
The mobile non-invasive solution for liver fibrosis assessment
Powered by LSM by VCTE™ for liver fibrosis assessment.


FibroScan® Mini 430 is designed for multi-center sharing and remote liver patient management.
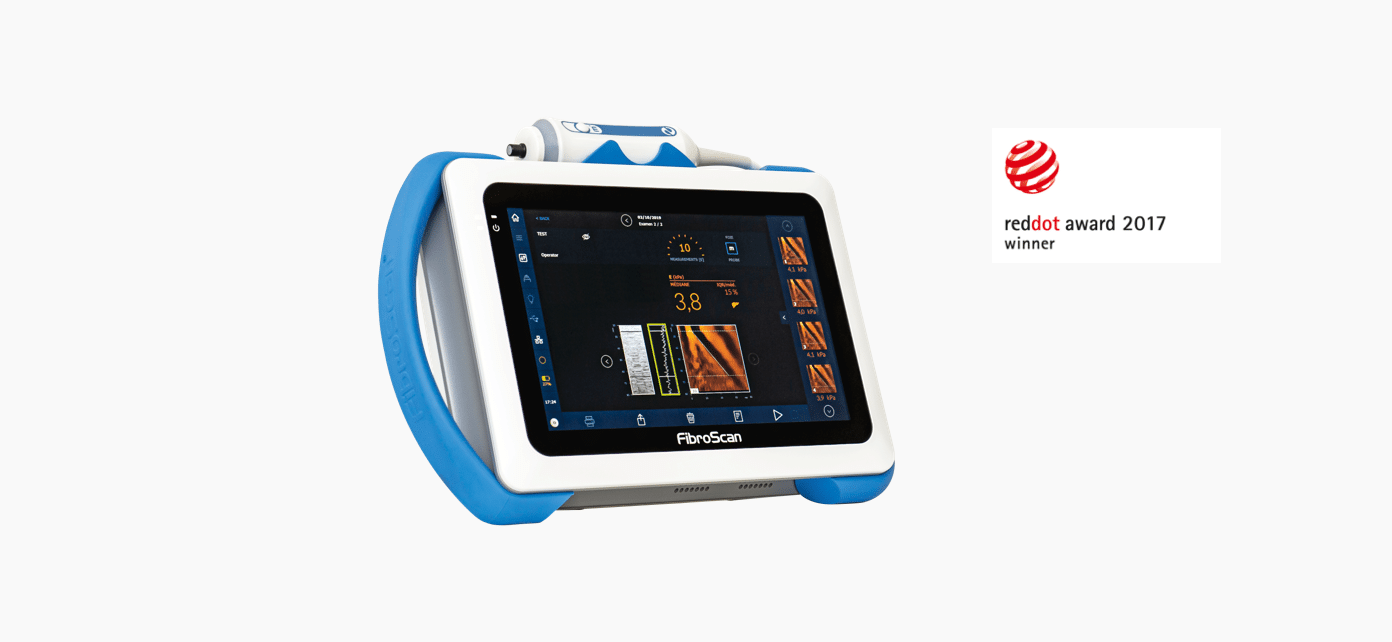
LSM by VCTE™ is unique, patented and validated for liver fibrosis assessment. It is the standard for non-invasive evaluation of liver stiffness with 3,245 international peer-reviewed publications.1
Products in the FibroScan® range are Class IIa medical devices as defined by Directive 93/42/EEC (EC 0459). These devices are designed for use in a medical practice in order to measure liver stiffness and ultrasound attenuation in patients with liver disease. Examinations with FibroScan® device shall be performed by an operator who has been certified by the manufacturer or its approved local representative. Operators are expressly recommended to carefully read the instructions given in the user manual and on the labelling of these products. Check cost defrayal conditions with paying bodies.
References are available in our bibliography.