
FibroScan® by echosens
The non-invasive gold standard solution for comprehensive management of liver health
CAP™ is the non-invasive reference parameter used as an aid to diagnose and monitor liver steatosis.
CAP™ is available as an option.

There is a silent epidemic of fatty liver disease in the general population, called MASLD/MASH. The main causes of fatty liver disease are excessive alcohol consumption and/or poor diet and sedentary lifestyle.
If patients remain undiagnosed and untreated, they may go on to develop irreversible cirrhosis of the liver. If treated early with diet and lifestyle changes, the disease can be reversible.
Most patients with the presence of a metabolic syndrome.
Risk factors and predictors of MASLD
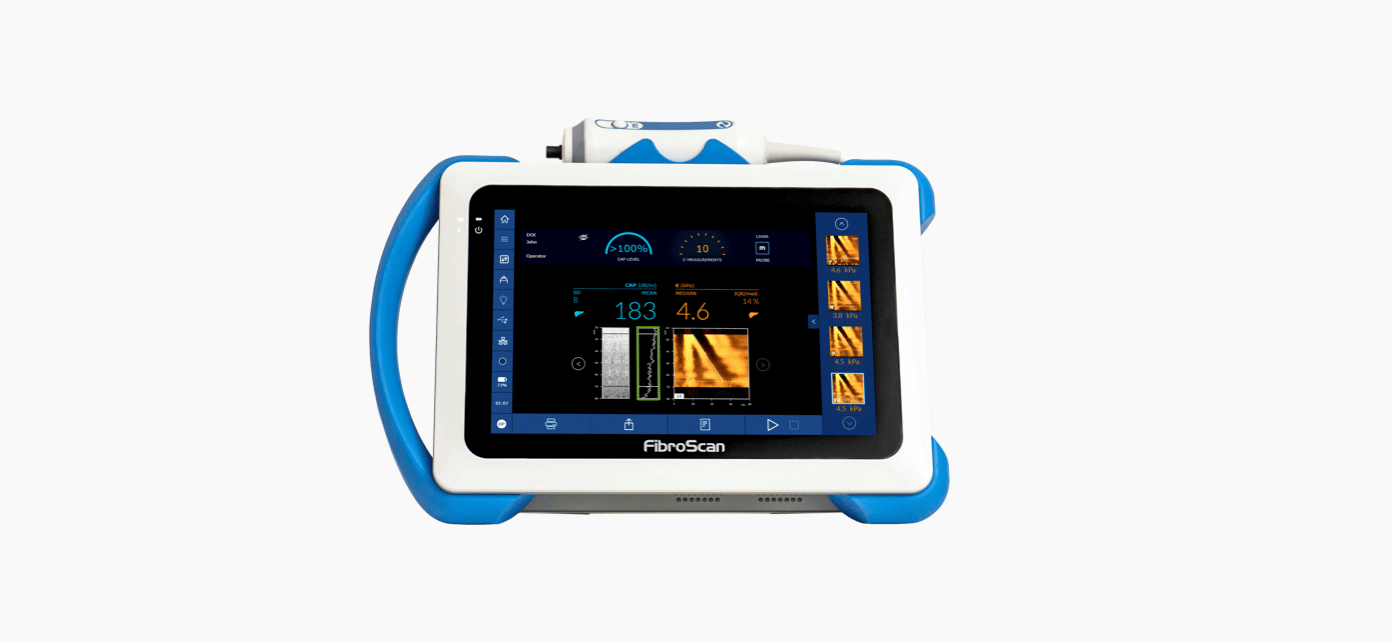
CAP™ is unique, patented and validated for liver steatosis assessment1,2: 1,130 international and peer-reviewed articles support the use of CAP™.
CAP™ is a guiding point for doctors and patients to improve monitoring of lifestyle change and therapeutic intervention.
CAP™ is a quantitative surrogate of liver steatosis expressed in decibel per meter (db/m).
Both LSM by VCTE™ and CAP™ are measured simultaneously without lengthening the examination time.
CAP™ is available on the three FibroScan® probes (S+*, M+ and XL+).
*CAP™ is not available on S+ probe in all countries.
1. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022-1030. doi:10.1016/j.jhep.2016.12.022.
2. Recio E, Cifuentes C, Macías J, et al. Interobserver concordance in controlled attenuation parameter measurement, a novel tool or the assessment of hepatic steatosis on the basis of transient elastography. Eur J Gastroenterol Hepatol. 2013;25(8):905-911.doi:10.1097/MEG.0b013e32835f4c3d.
Products in the FibroScan® range are Class IIa medical devices as defined by Directive 93/42/EEC (EC 0459). These devices are designed for use in a medical practice in order to measure liver stiffness and ultrasound attenuation in patients with liver disease. Examinations with FibroScan® device shall be performed by an operator who has been certified by the manufacturer or its approved local representative. Operators are expressly recommended to carefully read the instructions given in the user manual and on the labelling of these products. Check cost defrayal conditions with paying bodies.

The non-invasive gold standard solution for comprehensive management of liver health

Seamless liver health assessment for all

Cloud-based solution to assist clinicians in providing comprehensive liver care.

Enhance FibroScan® liver disease assessment with biological markers